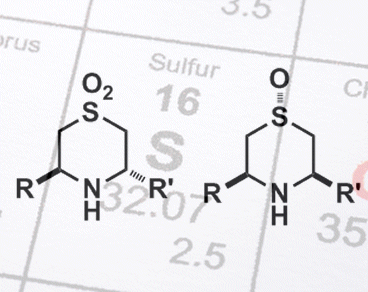
Chiral 3,5-disubstituted 1,4-thiazane S-oxides – compounds containing a 6-membered ring in which a sulfur atom, at position 1, sits opposite a nitrogen atom, at position 4, that has two pendent groups at positions 3 and 5 – are biologically important and have antioxidant, anticancer, and antibiotic properties. However, the stereoselective synthesis of these compounds is challenging.
Cyclization of a (homo-chiral) straight-chain amine, by using an addition reaction (intramolecular aza-Michael) can lead to two different stereoisomeric products. But by simply altering the oxidation state of the sulfur atom present, Stefan Söderman and Adrian Schwan, University of Guelph, Canada, discovered they could select for a single product. Starting amines containing a sulfoxide group (R–S(=O)–R’) gave a trans product, and those with a sulfone group (R-S(=O)2-R’) gave a cis product. Both reactions were efficient (>90 % yields) and highly selective (typically >90 %). Sulfoxides are capable of forming good intramolecular H bonds, whereas sulfones are not. This difference, likely accounts for the different products owing to different induced transition states. These mechanistic differences are currently under investigation.
- Stereodivergent Access to Cis-and Trans-3,5-Disubstituted 1,4-Thiazane 1-Oxides by Cyclization of Homochiral β-Amino Sulfoxides and Sulfones. The Preparation of Isomeric Ant Venom Alkaloids,
Stefan C. Söderman, Adrian L. Schwan,
Org. Lett. 2013.
DOI: 10.1021/ol4023003