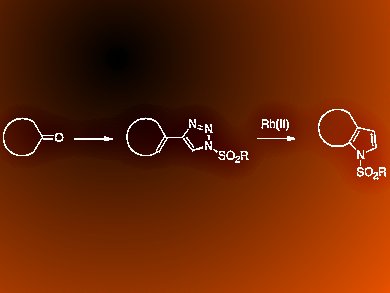
Pyrroles are used in a wide variety of natural products, therapeutic agents, and high-value materials. However, the synthesis of highly functionalized pyrroles remains challenging. It often requires multiple steps or harsh reaction conditions.
Huw M. L. Davies, Joshua S. Alford, and Jillian E. Spangler, Emory University, Atlanta, Georgia, USA, describe the synthesis of 2,3-fused pyrroles via an intramolecular rhodium(II)-catalyzed cyclization of 4-alkenyl-N-sulfonyltriazoles, which are readily derived from the corresponding ketones. The rhodium-catalyzed reaction of 4-alkenyl-1-sulfonyl-1,2,3-triazoles features an unusual 4π electrocyclization.
The substrate scope of this transformation is broad and the products are formed under mild reaction conditions. The team extend the methodology to the formation of substituted indoles from cyclohexanones in a one-pot reaction starting from 1-ethynylcyclohexenes.
This novel transformation contrasts with the vast majority of reactions of N-sulfonyltriazoles, which involve intermolecular reactions of the iminocarbene intermediates.
- Conversion of Cyclic Ketones to 2,3-Fused Pyrroles and Substituted Indoles,
Joshua S. Alford, Jillian E. Spangler, Huw M. L. Davies,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja405043g