Recent progress in flow-chemistry techniques has opened up a wide range of opportunities in organic synthesis. Continuous production of target compounds with minimal purification by means of supported reagents is very attractive.
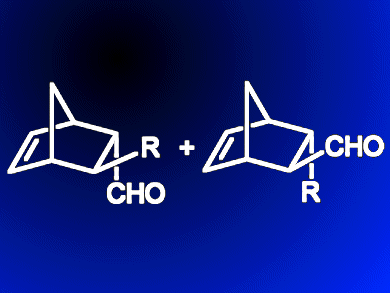
Maurizio Benaglia, Alessandra Puglisi and colleagues, Università degli Studi di Milano, Italy, have made a “homemade” HPLC column that performs Diels–Alder cycloaddition reactions under continuous-flow conditions with an organocatalyst bound to a solid support. The organocatalysts — salts of an imidazolidinone compound — were readily immobilized on standard laboratory silica before being packed into a column. The cycloaddition reaction between cyclopentadiene and three simple aldehydes was then studied. By altering the different catalyst salts and flow rates, cycloadducts (with four stereocentres) could be achieved in >95 % yield with good stereoselectivity (ee >83 %) at room temperature. This versatile set-up also allows different reactions to be run sequentially over long periods of time (>150 h). Straightforward regeneration of the catalyst then further extends operating times to >300 h without an appreciable drop in product quality or yield.
- Continuous-Flow Stereoselective Organocatalyzed Diels–Alder Reactions in a Chiral Catalytic “Homemade” HPLC Column
Valerio Chiroli, Maurizio Benaglia, Franco Cozzi, Alessandra Puglisi, Rita Annunziata, Giuseppe Celentano
Org. Letters 2013.
DOI: 10.1021/ol401390z