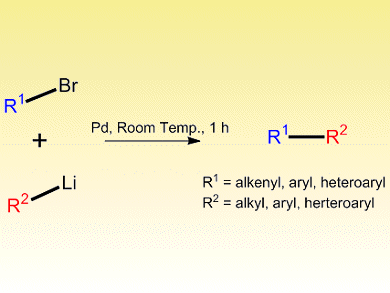
Carbon–carbon bond forming reactions catalyzed by cross-coupling reactions are key to numerous synthetic protocols. Although coupling reactions that use organometallic reagents are extremely popular, the high reactivity and poor selectivity of common organolithium reagents have largely prohibited their use.
Ben Feringa and colleagues from the University of Groningen, The Netherlands, have now developed a practical and general method for the fast and highly selective direct cross-coupling of organolithium reagents. By using a Pd-based catalyst, Pd[P(tBu)3]2, a wide range of alkyl-, aryl- and heteroaryl-lithium reagents selectively couple with aryl- and alkenyl-bromides to give synthetically useful intermediates. Tuning of the reaction conditions, including type and volume of solvent, and amount of catalyst and whether it was generated commercially or in situ, resulted in excellent rates of conversion and selectivity.
The process proceeds quickly at room temperature and crucially avoids lithium halogen exchange and homocoupling. Halide, acetal, ether, amine and alcohol groups all tolerate the reaction conditions, and importantly, organolithium reagents are often cheap or readily synthesized.
- Direct catalytic cross-coupling of organolithium compounds,
Massimo Giannerini, Martín Fañanás-Mastral, Ben L. Feringa,
Nat. Chem. 2013.
DOI: 10.1038/nchem.1678