There is an abundance of natural products to be studied from marine organisms, many of which have novel physiological properties that translate into antibiotic, anticancer, anti-inflammatory, and other activities of interest to medicinal chemists. However, many of these substances are complex and offer numerous challenges for synthetic chemists.
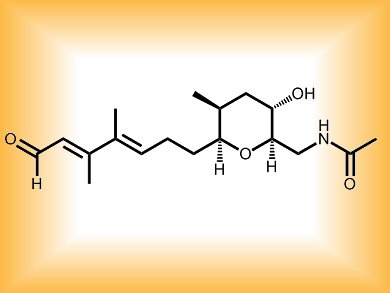
One such compound, (–)-brevisamide (pictured), a toxin from the “red tide” dinoflagellate Karenia brevis, provides a potential model for how fused polyethers are biosynthesized. Researchers at the University of Hyderabad, India, and their colleagues at the CSIR-Indian Institute of Chemical Technology, Hyderabad, have devised a formal stereoselective synthesis of the monocyclic ether alkaloid (–)-brevisamide. The team worked their way through a series of synthetic trials to come up with a reaction scheme involving a syn-Aldol reaction, Sharpless asymmetric epoxidation, and stereoselective construction of a tetrahydropyran ring by 6-endo-cyclization of a substituted hydroxy styrylepoxide.
- A formal stereoselective synthesis of (–)-brevisamide,
J. S. Yadav, A. Raju, K. Ravindar, B. V. Subba Reddy,
Tetrahedron Lett. 2013, 54, 3227–3229.
DOI: 10.1016/j.tetlet.2013.01.134