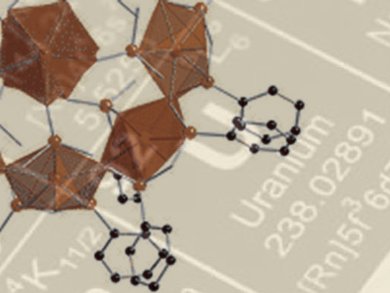
Uranyl nanotubules and nanospheres have been investigated for several years as part of the chemical quest for materials for use in advanced nuclear energy systems. However, until now synthesis of discrete compounds of uranyl–organics that form cages or clusters has not been particularly fruitful.
The in situ synthesis of hydrogen arsenate and pyroarsonate ligands has given Peter Burns and colleagues, University of Notre Dame, Indiana, USA, the flexibility to access a hybrid uranyl cage through self assembly. The team has used a hybrid approach to induce the required geometry without peroxides. It has successfully made uranyl diphosphonate nanotubules and a bimetallic uranyl heteropolyoxometalate cage. They thus demonstrated that monomeric uranyl cations are able to be connected through flexible arsonate derivatives to make uranyl nanospheres.
- Hybrid Uranyl Arsonate Coordination Nanocages,
Pius O. Adelani, Ginger E. Sigmon, Peter C. Burns,
Inorg. Chem. 2013.
DOI: 10.1021/ic400827h