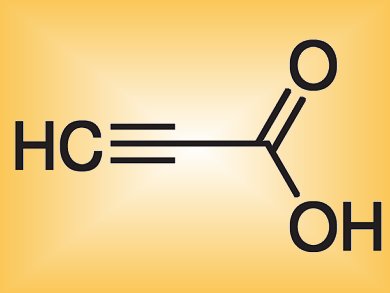
Propargylic compounds are very versatile building blocks in organic synthesis. The C–C bond forming reaction between an alkyne and a carbonyl group to give these triply bonded compounds is consequently important. Although a range of syntheses exists, many require expensive reagents or harsh reaction conditions.
Yoshinori Kondo and colleagues, Tohoku University, Japan, have developed a method that directly incorporates CO2 into alkynylsilanes by using cesium fluoride to give various propiolic acids under ambient conditions. CO2 benefits from being readily available, inexpensive, and non-toxic.
The team studied each reaction component in turn to optimize the conditions, and found that polar solvent dimethyl sulfoxide (DMSO) and base CsF work the best. Both aryl and alkyl substituted alkynylsilanes reacted smoothly with good to excellent yields. The system was also tolerant of hydroxyl, halo, cyano, and nitro functional groups.
Further studies to extend the scope of the process are underway.
- Carboxylation of alkynylsilanes with carbon dioxide mediated by cesium fluoride in DMSO,
M. Yonemoto, K. Inamoto, Y. Tanaka, Y. Kondo,
Org. Biomol. Chem. 2013.
DOI: 10.1039/C3OB40760H