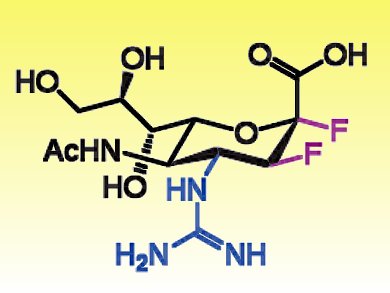
Neuraminidase is a sialic acid hydrolase that promotes viral spreading. Widely used anti-influenza drugs, such as zanamivir and oseltamivir, selectively inhibit neuraminidase. Some influenza strains, however, are resistant to these therapies.
To overcome this limitation, Jin-Hyo Kim, University of British Columbia, Canada, and colleagues developed a novel class of drugs. The researchers demonstrated that the reaction catalyzed by the viral neuraminidase involves the formation of a covalent intermediate. This intermediate can be exploited to block the viral replication. Difluorosialic derivatives bearing an equatorial fluorine at C2 and guanine substituents at C4 (such as FeGuDFSA, pictured) stabilized the covalent intermediate and were as efficient as zanamivir in blocking the viral replication.
These compounds, moreover, showed a broad-spectrum activity against viral strains resistant to zanamivir and oseltamivir. Therefore, they represent novel promising anti-influenza drugs.
- Mechanism-Based Covalent Neuraminidase Inhibitors with Broad-Spectrum Influenza Antiviral Activity,
J. H. Kim, R. Resende, T. Wennekes, H. M. Chen, N. Bance, S. Buchini, A. G. Watts, P. Pilling, V. A. Streltsov, M. Petric, R. Liggins, S. Barrett, J. L. McKimm-Breschkin, M. Niikura, S. G. Withers,
Science 2013, 340 (6128), 71–75.
DOI: 10.1126/science.1232552