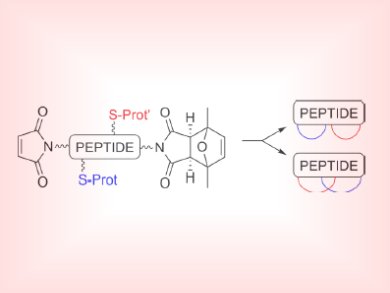
Cyclization is an established method to increase the stability of a peptide toward enzymatic degradation and allow for preorganization of functional groups and better cell permeability. Although a number of methods exist to prepare cyclic peptides, few take advantage of the familiar synthetic organic strategy, the click reaction, between a thiol and maleimide to obtain cyclic biomolecules.
Anna Grandas and colleagues, Universitat de Barcelona, Spain, took a short cysteine-containing peptide sequence (KYAYCG assembled on Rink amide resin) with maleimide side groups and created cyclic peptides by using a thio-ene Michael reaction as the key step. By combining both free and protected maleimide groups into the peptide sequence, two structurally different bicyclic peptides could be formed as either isolated (spectacle-like) or fused cycles.
Although small amounts of larger macrocycles, dimers and trimers, were formed as side-products, this method was shown to be suitable to synthesize up to 43-membered rings.
- Straightforward Synthesis of Cyclic and Bicyclic Peptides,
Xavier Elduque, Enrique Pedroso, Anna Grandas,
Org. Lett. 2013.
DOI: 10.1021/ol400726y