Researchers at the Federal University of São Carlos, Brazil, explain that co-crystals of pharmaceutical products almost always have different physical properties than the isolated drug, such as improved water solubility and thus better oral availability. However, preparing co-crystals often requires toxic organic solvents.
Frederico Soares and Renato Carneiro have now demonstrated the co-crystallization of the non-steroidal anti-inflammatory analgesic ibuprofen, with nicotinamide as the conformer, in an aqueous solution. The team adhered to green chemistry principles, finding an approach that works at the low reaction temperature of 60 °C and avoids the use of organic solvents. They were able to track the preparation using inline Raman spectroscopy as water produces practically no Raman scattering.
- Green Synthesis of Ibuprofen–Nicotinamide Cocrystals and In-Line Evaluation by Raman Spectroscopy,
Frederico L. F. Soares, Renato L. Carneiro,
Cryst. Growth Des. 2013.
DOI: 10.1021/cg3017112
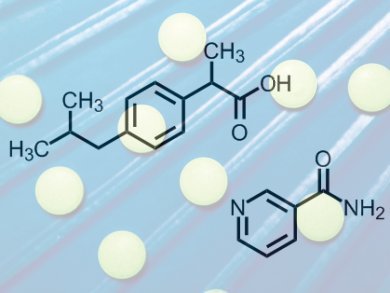



The structure of ibuprofen is depicted wrongly in the picture. The termina a CO-OH is shown as CO-CH3. It should be rectified immediately.
You are quite right, the original picture for this article did indeed contain a mistake. We have corrected the structure, thank you for pointing out our error. The ChemViews team.