Allenes are important synthetic intermediates in organic chemistry and biologically active molecules. Allenes have three carbon atoms connected by adjoining carbon–carbon double bonds that can make them axially chiral and often difficult to synthesize. However, a general strategy to synthesize fully substituted allenes by using asymmetric phase-transfer catalysis has been developed.
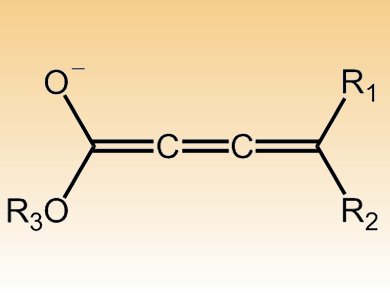
The method by Keiji Maruoka and colleagues, Kyoto University, Japan, uses cumulenolate, with three connected carbon–carbon double bonds (pictured), and a chiral phase-transfer catalyst to produce complex allenes.
An alleno-Mannich-type reaction and an alkylation reaction were studied. The best catalyst for the former was a 3,3′-disubstituted binaphthyl-based quaternary ammonium salt that gave an allene with a 90:10 diastereomeric ratio and 90 % enantiomeric excess (e.e.). For the latter, the catalyst was modified at the N-aryl group of the piperazine, and gave an 86:14 mix of regioisomers in 95 % e.e.
- Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes,
T. Hashimoto, K. Sakata, F. Tamakuni, M. J. Dutton, K. Maruoka
Nature Chem. 2013.
DOI: 10.1038/nchem.1567