Metal-centered enzymes are crucial for the oxidation and breakdown of a wide range of toxic compounds in our bodies. Researchers at the University of Lyon, France, and their colleagues have taken inspiration from the likes of cytochrome P-450 and soluble methane monooxygenase enzymes: They have used the iron heme and diiron non-heme units at the heart of these proteins to develop a model compound that catalyzes methane oxidation.
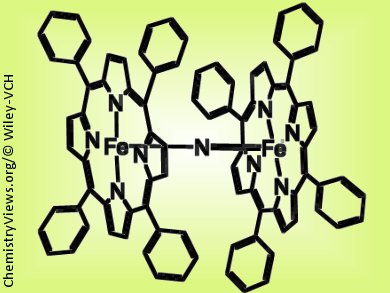
The researchers based their system on two iron-centered porphyrin rings that sandwich a bridging nitrogen atom between them (pictured). They characterized the resulting diiron oxo radical species formed at –90 °C using UV-Vis, electron paramagnetic resonance (EPR), and Mössbauer spectroscopy as well as electrospray ionization mass spectrometry. Tests show that this species can readily transfer oxygen atoms to alkanes including methane, which bodes well for the development of similar compounds as “green” oxidising agents for the functionalization of alkanes.
- An N-bridged high-valent diiron–oxo species on a porphyrin platform that can oxidize methane,
Evgeny V. Kudrik, Pavel Afanasiev, Leonardo X. Alvarez, Patrick Dubourdeaux, Martin Clémancey, Jean-Marc Latour, Geneviève Blondin, Denis Bouchu, Florian Albrieux, Sergey E. Nefedov, Alexander B. Sorokin,
Nature Chem. 2012.
DOI: 10.1038/nchem.1471