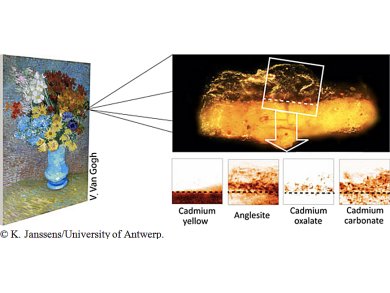
Geert Van der Snickt, University of Antwerp, Belgium, and colleagues show by X-ray analysis that some bright yellow flowers of the Van Gogh painting “Flowers in a blue vase” from 1887 have turned to an orange-grey color over time, due to a supposedly protective varnish applied after the master’s death.
Cadmium yellow (CdS) oxidizes in unvarnished paintings with air to CdSO4 making the pigments lose color and luminosity. However, two microscopic paint samples studied using powerful X-ray beams at the European Synchrotron Radiation Facility (ESRF), Grenoble, France, and at Deutsches Elektronen-Synchrotron (DESY)’s PETRA III in Hamburg, Germany, did not show CdSO4. The sulphate anions reacted with the lead ions from the varnish and had formed anglesite (PbSO4), an opaque compound that was found nearly everywhere throughout the varnish. The source of the lead probably is a lead-based siccative that had been added to the varnish.
At the interface between paint and varnish, the cadmium ions together with degradation products from the varnish itself also formed a layer of cadmium oxalate (CdC2O4). Together with the anglesite, the CdC2O4 accounts for the opaque, orange-grey crust disfiguring parts of the painting on a macroscopic level.
The results illustrated once more that it is of great importance for conservators to know the materials present in their painting collection in order to trace and monitor risk pigments, detect degradation processes in an early stage, adjust the conservation treatment, and adapt the environmental conditions if necessary.
- Combined use of synchrotron radiation-based μ-XRF, μ-XRD, μ-XANES and μ-FTIR reveals to alternative degradation pathway of the pigment cadmium yellow (CdS) in a painting by Van Gogh,
G. van der Snickt et al.,
Analytical Chem. 2012.
DOI: 10.1021/ac3015627