The development of one of the most reliable C-C bond-forming reactions in organic synthesis, the Mukaiyama aldol reaction, is surveyed by Taku Kitanosono and Shu Kobayashi, University of Tokyo, Japan.
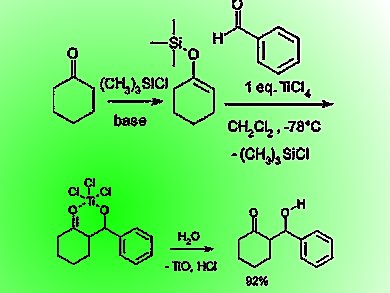
The original Mukaiyama aldol reactions were carried out with a silyl enol ether and an aldehyde using stoichiometric amounts of Lewis acids such as TiCl4 in organic solvents under strictly anhydrous conditions (pictured). Efforts to develop the reaction in aqueous media led to many fruitful scientific advances not only in organic chemistry and organic synthesis but also in inorganic chemistry and biochemistry.
For example, in 1991 it was found that Mukaiyama aldol reactions proceeded under milder conditions and obtained high yields with wide substrate scope in the presence of a catalytic amount of lanthanide triflates in aqueous solvents. This finding put aside the former believes that Lewis acids decompose rapidly even in the presence of small amounts of water. This lead to the discovery of a series of water-compatible Lewis acids.
The chemistry in this field is still being actively pursued. Compared with the well-refined catalytic systems for asymmetric hydroxymethylation in aqueous media, contemporary catalysts for reactions between hydrophobic substrates have the fault of substrate specificity and lack broader catalytic activities.
- Mukaiyama Aldol Reactions in Aqueous Media,
Taku Kitanosono, Shu Kobayashi,
Adv. Synth. Catal. 2013.
DOI: 10.1002/adsc.201300798
This review is published in celebration of the 40th anniversary of the Mukaiyama Reaction.