Halolactonization of unsaturated carboxylic acids is widely used in organic synthesis. Unfortuantely, iodonium and bromonium ions tend to readily racemize, which makes it difficult to control stereochemistry during the synthesis of compounds of biological interest, for instance.
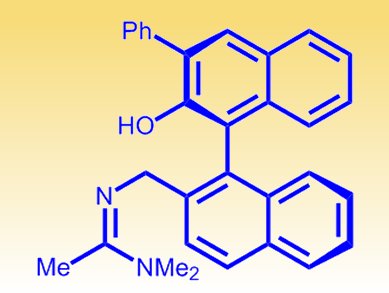
Researchers at the University of Texas, USA, have modified BINOL (1,1′-bi-2-naphthol) to create a bifunctional catalyst (pictured) that takes control of bromine, allowing chemists to carry out bromolactonizations of diverse unsaturated acids. The team reports for the first time a catalytic desymmetrization of a prochiral dienoic acid using their enantioselective bromolactonization approach.
- Bifunctional Catalyst Promotes Highly Enantioselective Bromolactonizations To Generate Stereogenic C–Br Bonds,
Daniel H. Paull, Chao Fang, James R. Donald, Andrew D. Pansick, Stephen F. Martin,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja305117m