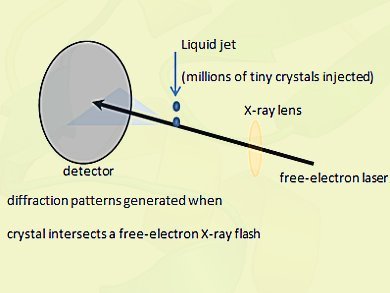
Ilme Schlichting, Max Planck Institute for Medical Research, Heidelberg, Germany, and international colleagues analyzed tiny protein crystals using short pulses of X-ray light from the first hard X-ray free-electron laser, the US Department of Energy’s 300 million dollar Linac Coherent Light Source at Stanford.
X-ray free-electron lasers are extremely powerful new X-ray sources that provide highly intense ultrashort flashes of light. The intensity of such an X-ray pulse is more than a billion times higher than that provided by the most brilliant state-of-the-art X-ray sources. It has a thousand-fold shorter pulse length, on the order of a few millionths of a billionth of a second, or femtoseconds.
The ultra-short pulses can pass through the sample before the tiny crystals are completely destroyed by the intensity of the laser and thus provide the necessary scattering signal of the still-intact molecules.
This structural analysis reveals details with a spatial resolution of 0.2 millionth of a millimeter. The team showed that their data compared well with those collected from large, well-characterized crystals using conventional X-ray sources, providing a benchmark for the new free-electron laser approach.
The free-electron laser is an important new tool for structural biology on large macromolecular assemblies and membrane proteins, many of which are known to be important targets for pharmaceutical development. Since small crystals are typically easier to produce than large ones, this is of immediate relevance for all studies of molecules that are difficult to crystallize.
- Max Planck Gesellschaft, Munich, Germany