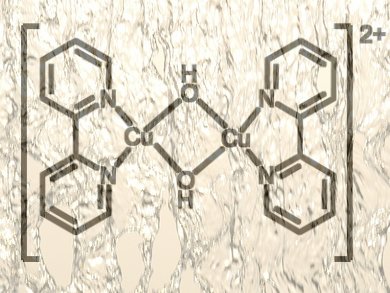
Chemists led by Karen Goldberg, University of Washington, Seattle, USA, have taken a step towards an efficient, robust, and inexpensive catalyst that can release oxygen from water. The research could eventually pave the way towards overcoming the key problems of obtaining fuels electrochemically. The team was able to generate copper–bipyridine–hydroxo complexes quickly and easily from commercially available copper salts and bipyridine at high pH. A 750 millivolt over-potential is all that is required to release oxygen from water electrochemically using this catalyst. The team says this is one of the fastest of such catalysts with a turnover frequency of about 100 s–1.
- A soluble copper–bipyridine water-oxidation electrocatalyst,
S. M. Barnett, K. I. Goldberg, J. M. Mayer,
Nature Chem. 2012.
DOI: 10.1038/nchem.1350