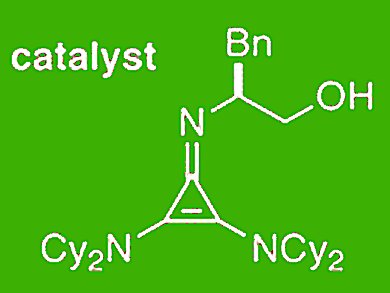
Of particular interest in recent years has been the development of chiral Brønsted bases capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products. Jeffrey S. Bandar and Tristan H. Lambert, Columbia University, New York, USA, show that cyclopropenimines are a highly effective new class of enantioselective Brønsted base catalysts.
A chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. The reseachers demonstrate a preparative scale reaction to deliver 25 g of product, show a trivial large scale synthesis of the optimal catalyst, and meausre for the first time the basicity of a 2,3-bis(dialkylamino)cyclopropenimine. It is approximately equivalent to the P1–tBu phosphazene base.
The exceptional performance of the chiral cyclopropenimine versus related guanidine bases suggests that these new catalysts may enable important developments in the area of enantioselective Brønsted base catalysis. The easy preparation of cyclopropenimines and their amenability to use on a multigram scale should make them suitable to a range of applications.
- Enantioselective Brønsted Base Catalysis with Chiral Cyclopropenimines,
Jeffrey S. Bandar, Tristan H. Lambert,
J. Am. Chem. Soc. 2012, 134, 5552-5555.
DOI: 10.1021/ja3015764