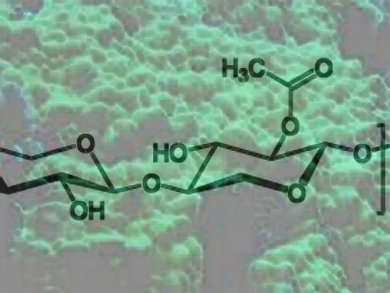
Algae produce a considerable number of unusual polysaccharides that are not found in higher plants. A number of algae produce cell walls that are completely devoid of cellulose. Many of these cellulose-free algae rely on other polysaccharides like 1,3-xylan, a homopolysaccharide of β-1,3-linked D-xylopyranose units, which is found in some red and siphonous green algae.
Xylanases capable of degrading the crystalline microfibrils of 1,3-xylan that reinforce the cell walls of these green algae have not been well studied. To gain a better mechanistic understanding of these enzymes, Stephen G. Withers, University of British Columbia, Vancouver, Canada, and colleagues from Japan synthesized a suite of reagents and evaluated thier function as substrates and inhibitors of an endo-1,3-xylanase.
By this a retaining mechanism was confirmed for the xylanase, its catalytic nucleophile identified, and the existence of −3 to +2 substratebinding subsites demonstrated. Protein crystal X-ray diffraction methods provided a high resolution structure of a trapped covalent glycosyl−enzyme intermediate, indicating that the 1,3-xylanases likely utilize the 1S3 → 4H3 → 4C1 conformational itinerary to effect catalysis.
The findings are usefull for algaculture and the production of renewable chemical commodities.
- Mechanistic Insights into the 1,3-Xylanases: Useful Enzymes for Manipulation of Algal Biomass,
Ethan D. Goddard-Borger, Keishi Sakaguchi, Stephan Reitinger, Nobuhisa Watanabe, Makoto Ito, Stephen G. Withers,
J. Am. Chem. Soc. 2012.
DOI: 10.1021/ja211836t