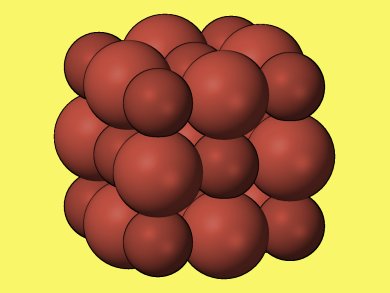
Researchers around B. Kiran, McNeese State University, Louisiana, USA, found through theoretical calculations based on density functional theory that (PbS)32 is the smallest stable cubic cluster that possesses both the same cubic structure and coordination number as the bulk crystal. Lead sulfide (PbS) forms when an equal number of lead and sulfur atoms exchange electrons and bond together in cubic crystals.
Cubic (PbS)32 is the smallest cluster, exhibiting sixfold coordination, that can be replicated to obtain the bulk crystal.
The crystal was identified by running computer simulations that calculated the energy and geometry of different structures containing different numbers of atoms. By using scanning tunneling microscope images to measure the dimensions of the resultant lead sulfide nano-blocks, the researchers confirmed that the (PbS)32 “baby crystals” had indeed stacked together as theoretically predicted.
- (PbS)32: A baby crystal,
B. Kiran, Anil K. Kandalam, Rameshu Rallabandi, Pratik Koirala, Xiang Li, Xin Tang, Yi Wang, Howard Fairbrother, Gerd Gantefoer, Kit Bowen,
J. Chem. Phys. 2012, 136, 024317.
DOI: 10.1063/1.3672166