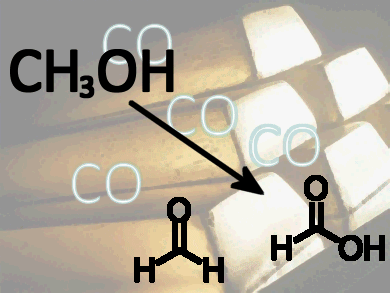
Oxidation of methanol to formaldehyde and formic acid is an important step in the development of novel fuels and numerous fine chemical products. Chemists usually try to exclude carbon monoxide from heterogeneous catalytic and electrocatalytic processes used in the oxidation of alcohols as it poisons the catalyst.
Researchers at Leiden University, The Netherlands, have exploited the surprising capacity of a gold electrocatalyst to adsorb CO irreversibly and to act as a promoter of the oxidation of alcohols in aqueous alkaline media, and of methanol in particular. The CO significantly lowers the onset potential for catalysis in this reaction, leading to enhanced formation of the main methanol oxidation products.
- The promoting effect of adsorbed carbon monoxide on the oxidation of alcohols on a gold catalyst,
P. Rodriguez, Y. Kwon, M. T. M. Koper,
Nature Chem. 2012.
DOI: 10.1038/nchem.1221