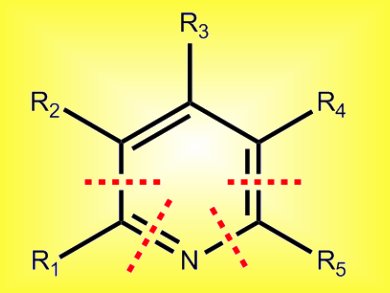
Aromatic nitrogen heterocycles play prominent roles in natural products and pharmaceutical agents. Of these, substituted pyridines are particularly abundant. While many chemical methods exist for the preparation of these heteroaromatics, the search for new strategies that offer concise and regiospecific access remain a topic of considerable interest.
Ming Chen and Glenn Micalizio, Scripps Research Institute, USA, have reported a highly convergent pyridine synthesis that proceeds from readily available starting materials. They used nucleophilic addition of a dithiane anion to an α,β-unsaturated carbonyl. The resulting allylic alcohol then underwent a metallacycle-mediated union with preformed trimethylsilane-imines. These imines were generated in situ by the low-temperature reaction of lithium hexamethyldisilazide with an aldehyde. Finally, a Ag(I)- or Hg(II)-mediated ring closure was used to form the pyridine.
This route offers great flexibility in the nature and substitution of pyridines that can be obtained: A range of di- through penta-substituted pyridines was formed with complete regiochemical control.
- Three-Component Coupling Sequence for the Regiospecific Synthesis of Substituted Pyridines
M. Z. Chen, G. C. Micalizio,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja2105703