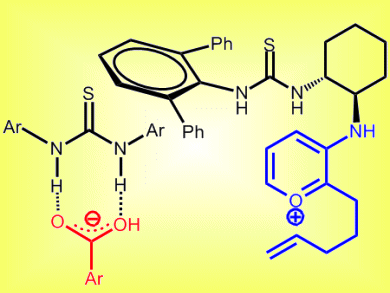
US researchers have used a dual catalyst consisting of a chiral primary aminothiourea and a second achiral thiourea to effect enantioselective intramolecular [5 + 2] cycloadditions based on oxidopyrylium intermediates. Their analysis of the reactions suggests that a new type of cooperative catalysis takes place in which each component is essential for generating a reactive pyrylium ion pair (pictured) which allows the subsequent cycloaddition to occur with high enantioselectivity.
[5 + 2] cycloadditions are widely used in organic synthesis so this approach offers a new approach to valuable tricyclic structures.
- Dual Catalysis in Enantioselective Oxidopyrylium-Based [5 + 2] Cycloadditions
N. Z. Burns, M. R. Witten, E. N. Jacobsen,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja206997e