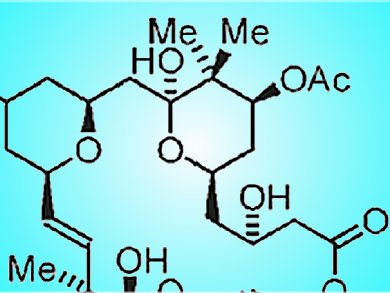
The bryostatins are a family of 20 marine natural products isolated from the bryozoan Bugula neritina. They have shown diverse biological effects including activity against Alzheimer’s disease, neural growth and repair, and the reversal of stroke damage. They have also been shown to restore apoptotic function and have other anti-cancer properties. As their natural abundance is insufficient to advance clinical studies, bryostatins have become a target for synthesis.
Michael J. Krische and co-workers, University of Texas at Austin, USA, have reported the most concise synthesis of a bryostatin to date. They synthesize bryostatin 7 (below) in 20 linear and 36 total steps. The key reaction is a hydrogen-mediated reductive coupling that forms C—C bonds. This reaction was employed in the formation of the core fragment A and to attach it to core fragment B (see below).

- Total Synthesis of Bryostatin 7 via C–C Bond-Forming Hydrogenation
Y. Lu, S. K. Woo, M. J. Krische,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja205673e