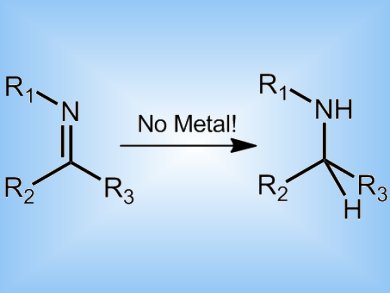
Organic catalysts that contain no expensive metal ions could open up new routes in synthetic chemistry according to researchers in Canada.
They have experimented with a fluorine-rich Lewis acid boron compound, tris(pentafluorobenzyl)boron, and have demonstrated that it can activate amines and so racemize chiral amines. By extension to a bimolecular reaction, the team has also shown that the same catalyst can effect hydride abstraction using the readily available compound diisopropylamine as the hydrogen source.
The catalyst opens up a new approach to metal-free stereochemical control in synthesis.
- Metal-Free Transfer Hydrogenation Catalysis by B(C6F5)3
J. M. Farrell, Z. M. Heiden, D. W. Stephan,
Organomet. 2011.
DOI: 10.1021/om2005832