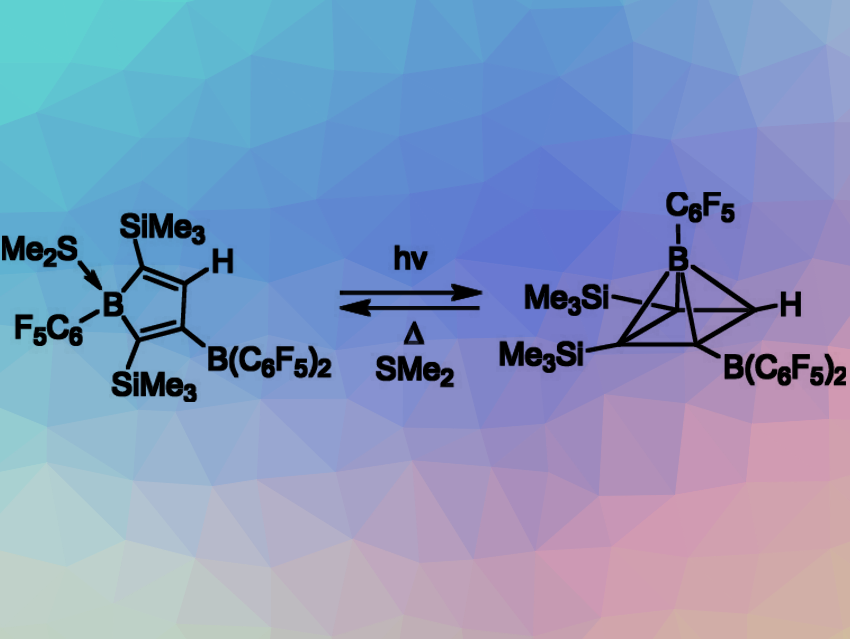
Gerhard Erker and colleagues, Westfälische Wilhelms-Universität Münster, Germany, have found that a suitably substituted borole (pictures left) can be readily and reversibly interconverted to its nonclassical borapyramidane isomer (pictures right). Boroles and borapyramidanes are classical and nonclassical constitutional isomers, respectively.
The borol adduct (pictured left) was prepared by the reaction of bis(alkynyl)B(C6F5)SMe2 with Piers’ borane [HB(C6F5)2]. This two-step sequence included an initial rare example of intermolecular alkyne 1,1-hydroboration, followed by intramolecular alkenyl boronation to close the five-membered boronol framework.
Photolysis of this classical borole adduct led in high yield to its nonclassical structural isomer, borapyramidane (pictured right). This neutral borapyramidane is a rare example of an isosteric of the (CH)5+ pyramidane cation. Thermolysis in the presence of SMe2 at 60 °C again formed the borole SMe2. At 100 °C, this molecule converted to an 2,3-bis-silyl-substituted borole isomer SMe2. Its photolysis also gave the borapyramidane. Prolonged photolysis of the borapyramidane at elevated temperatures converted it to a borapyramidane isomer containing a pair of trimethylsilyl groups in the 1,3-position.
- Borole/Borapyramidane Relationship,
Qiu Sun, Constantin G. Daniliuc, Xiaoye Yu, Christian Mück-Lichtenfeld, Gerald Kehr, Gerhard Erker,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c01727


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
