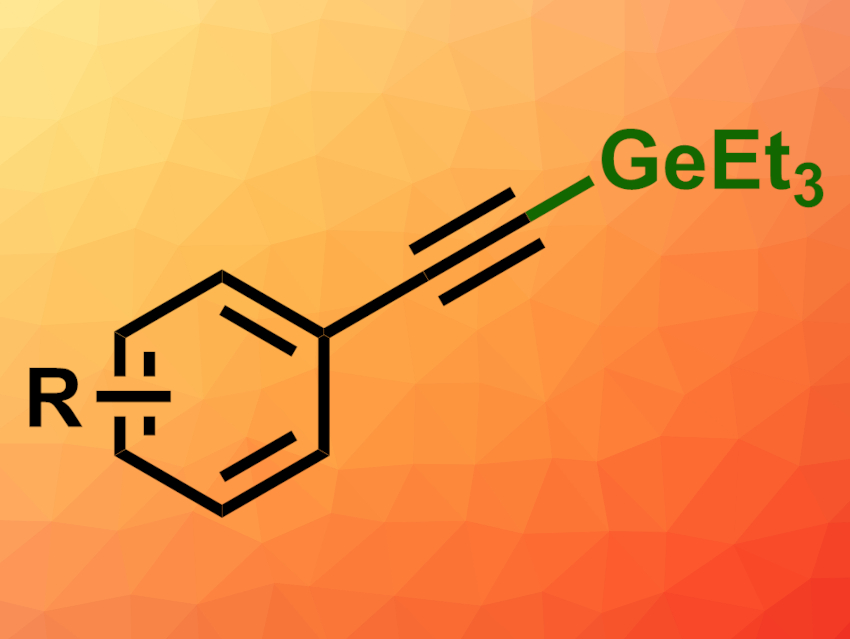
Organogermanes can be useful, e.g., in organic synthesis and catalysis. Methods for the formation of C–Ge bonds are, thus, interesting research targets. Access to alkynylgermanes, in particular, could still be improved.
Amit Dahiya and Franziska Schoenebeck, RWTH Aachen University, Germany, have developed a direct C(sp)–H germylation of terminal alkynes with triethyl germanium hydride (Et3GeH). The team used B(C6F5)3 as a Lewis acid catalyst and 2,6-lutidine as an organic base. They reacted different phenylacetylene derivatives with Et3GeH in toluene at 100 °C.
The desired products (general structure pictured) were obtained in good yields. The reaction tolerates both electron-withdrawing and electron-donating groups at the aromatic ring. The team proposes a mechanism that involves a hydride transfer from Et3GeH to B(C6F5)3 to give [HB(C6F5)3]–, while the 2,6-lutidine stabilizes the Et3Ge+ cation. The cation can then attack the alkyne group, and 2,6-lutidine facilitates the deprotonation of the resulting intermediate, giving the germylated product. The catalyst is then regenerated and H2 is released.
- Direct C–H Dehydrogenative Germylation of Terminal Alkynes with Hydrogermanes,
Amit Dahiya, Franziska Schoenebeck,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00840