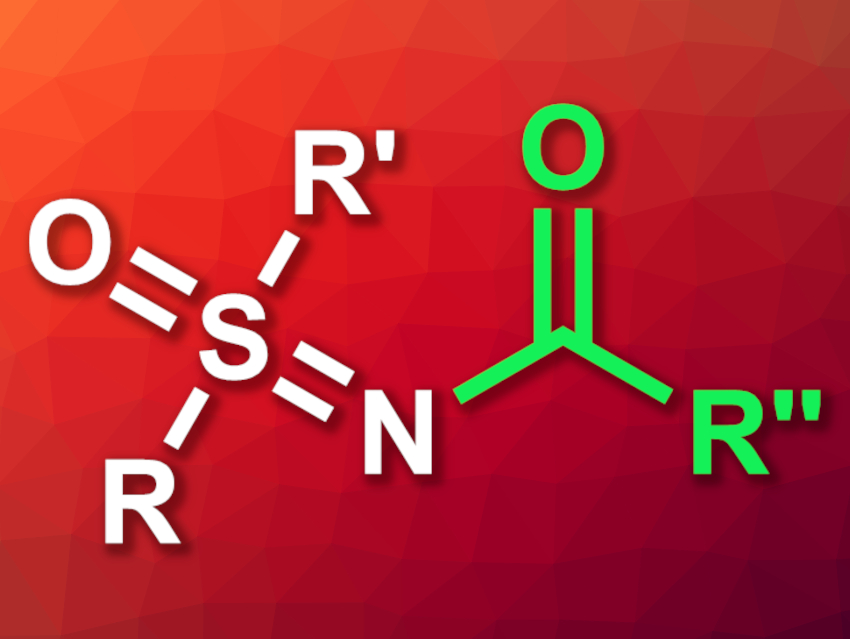
Sulfoximines (O=SR2=NH) are monoaza analogues of sulfones. They can be useful in organic synthesis, and there is a variety of reactions for the N-functionalization of sulfoximines—e.g., by alkylation, arylation, or acylation. N-acylation reactions of sulfoximines, however, often require activating agents or harsh conditions.
Wangze Song, Dalian University of Technology, China, and colleagues have developed a visible-light-induced, metal-, base-, and additive-free N-acylation of sulfoximines under mild conditions (general product structure pictured). The team reacted a range of thioacids (RC(=O)–SH) with different sulfoximines. They used 9-mesityl-10-methylacridinium tetrafluoroborate (Mes-Acr-MeBF4) as an organic photosensitizer and tetrahydrofuran (THF) or dimethyl sulfoxide (DMSO) as the solvent. The reactions were performed under air and a blue LED light at room temperature.
The desired N-acylated products were obtained in mostly good to excellent yields (up to 96 %). The approach can also be used to synthesize α-keto N-acyl sulfoximines using α-aroyl thioacids. It shows good functional group tolerance and compatibility with air.
- Visible-Light-Induced N-Acylation of Sulfoximines,
Pan Qiu, Xuelun Duan, Ming Li, Yubin Zheng, Wangze Song,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00843