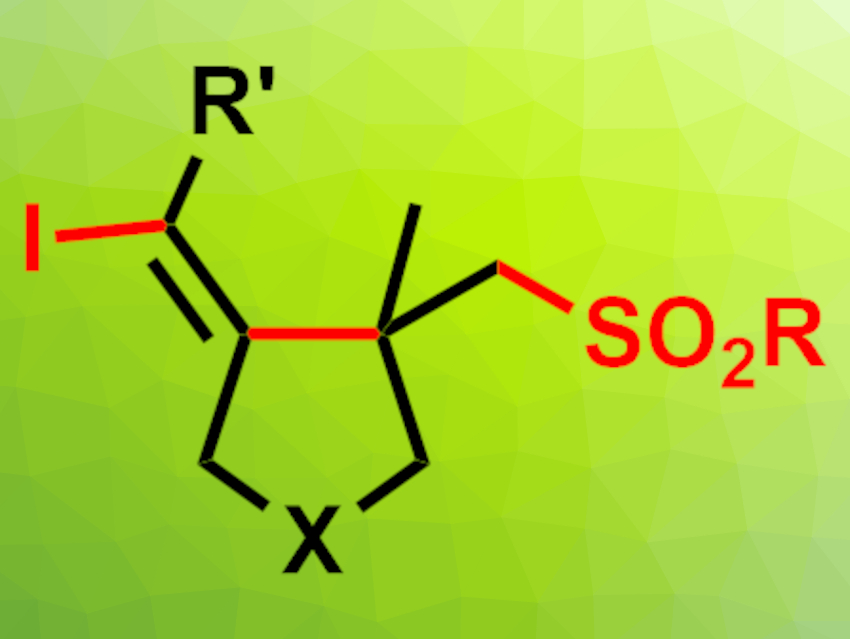
Sulfonated heterocycles can be useful, e.g., in pharmaceutical chemistry. To synthesize this type of compound, radical sulfonylative cyclizations can be used. However, they usually require stoichiometric oxidants or transition-metal catalysts.
Shaoqun Zhu, Yangzhou University, China, and colleagues have developed a radical iodosulfonylative cyclization reaction (product pictured, X = N,O,C) using a three-component system consisting of iodoform (CHI3), sodium sulfinates, and enynes that is performed under visible-light irradiation. The team reacted different enynes containing terminal alkene units with a range of sodium sulfinates and iodoform in MeCN at room temperature under blue LED irradiation.
The desired vinyl iodide- and sulfone-functionalized cyclic products were obtained in moderate to good yields. The reaction is performed under catalyst- and oxidant-free conditions. The team proposes a radical reaction mechanism in which a sulfonyl radical is generated by the reaction of the sodium sulfinate with iodoform under visible light. The sulfonyl radical reacts with the enyne in a radical addition, followed by a radical cyclization and iodine atom transfer.
- Visible-Light-Mediated Three-Component Radical Iodosulfonylative Cyclization of Enynes,
Qi Cheng, Fengrong Zhang, Xiaoyun Chen, Ying Han, Chaoguo Yan, Yaocheng Shi, Hong Hou, Shaoqun Zhu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00655