Organogermanium compounds have unique properties that can make them useful, e.g., in chemical synthesis, pharmaceuticals, and materials. However, compared with organosilicon and organotin compounds, organogermanium species are less well studied. One approach to the formation of C–Ge bonds is the catalytic addition of germanium hydrides to alkenes or alkynes. However, introducing a second functional group at the same time as a germyl fragment is challenging.
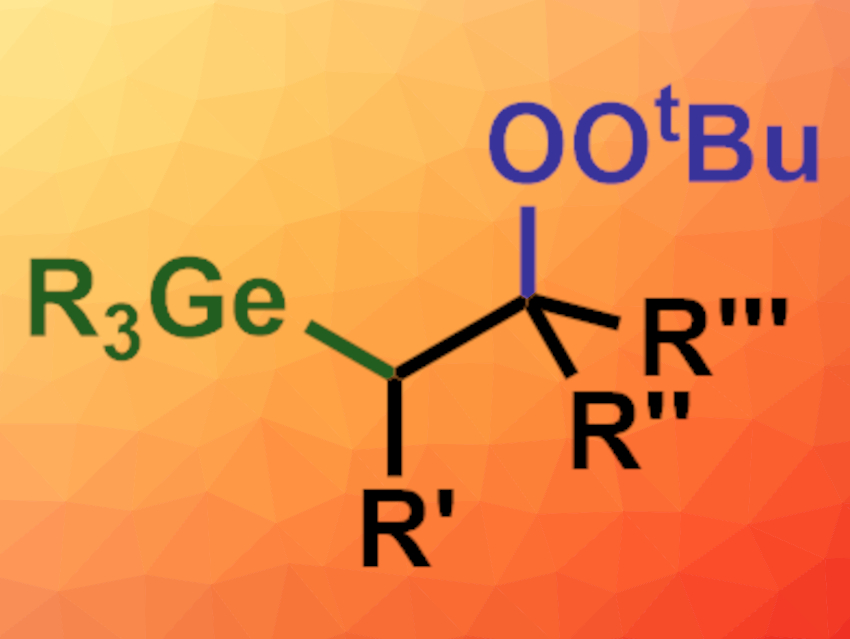
Leiyang Lv, Zhiping Li, Renmin University of China, Beijing, and colleagues have developed a method for the copper-catalyzed radical germyl peroxidation of alkenes (general product structure pictured). The team reacted a variety of alkenes with commercially available germanium hydrides, such as Ph3GeH, and tert-butyl hydroperoxide or cumene hydroperoxide. CuBr was used as the catalyst and MeCN as the solvent. The reactions were performed at 25 °C.
The desired difunctionalized products were obtained in generally good to excellent yields. Low yields were observed when using Ph2GeH2 or PhGeH3. The team proposes a radical reaction mechanism in which a single-electron transfer between Cu(I) and tert-butyl hydroperoxide generates a tert-butoxyl radical. This radical can then abstract the hydrogen atom of the germanium hydride to give a germanium-centered radical. The resulting species can react with the alkene, followed by a single-electron transfer to give an alkyl copper intermediate and then the desired product.
- Copper-Catalyzed Three-Component Germyl Peroxidation of Alkenes,
Yani Luo, Boxia Xu, Leiyang Lv, Zhiping Li,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00698