Phosphines are widely employed as ligands and can also be useful reactants in organic chemistry. Existing methods for the synthesis of phosphines have some drawbacks, e.g., limited substrate scopes or a need for harsh reagents or conditions. Using a deaminative reaction to synthesize phosphines using readily available alkylamines could be a promising alternative approach.
Penglei Cui, Chun Wang, Hebei Agricultural University, Baoding, China, Lipeng Wu, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, and colleagues have developed a deaminative alkylation of diphenyl phosphine or phenyl phosphine to give tertiary phosphines under visible-light photoredox catalysis. The team used different benzylamines, which were converted to triphenylpyridinium salts and then reacted with diphenyl phosphine or phenyl phosphine. Eosin Y was used as a photoredox catalyst and the reactions were performed under a white LED light. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was used as a base and MeCN as the solvent.
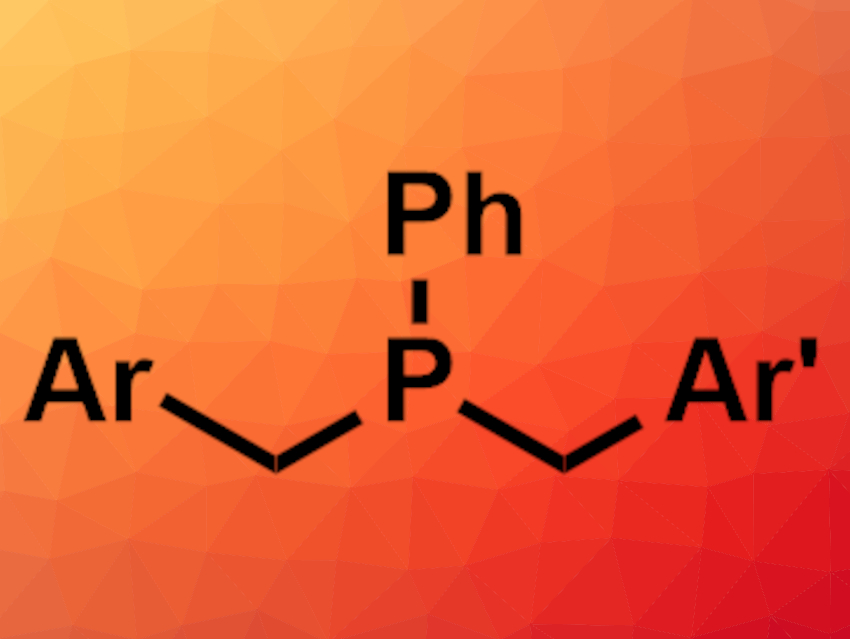
The desired complexes were isolated after complexation with BH3. The researchers found that two different alkyl groups could be introduced in a two-step reaction with phenyl phosphine, giving unsymmetrical tertiary phosphines. These compounds can be challenging to obtain otherwise. The approach could be useful to tune the electronic and steric properties of phosphine ligands.
- Visible-Light-Promoted Unsymmetrical Phosphine Synthesis from Benzylamines,
Penglei Cui, Sida Li, Xianjin Wang, Ming Li, Chun Wang, Lipeng Wu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00317