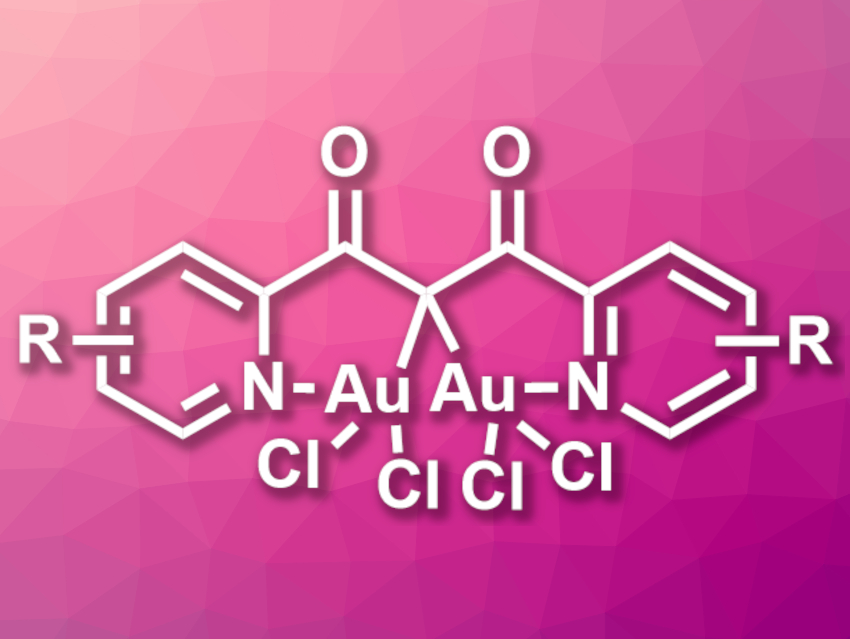
There are a variety of gem-diaurated gold(I) complexes with stabilizing aurophilic interactions between the Au(I) centers. Such complexes can be useful in catalysis. In contrast, gem-diaurated gold(III) complexes are rare and little is known about their catalytic properties. Existing gem-diaurated Au(III) complexes are based on phosphonium bis(ylide) ligands and do not allow for much structural variation.
Jonas F. Wunsch, Heidelberg University, Germany, A. Stephen K. Hashmi, Heidelberg University and King Abdulaziz University (KAU), Jeddah, Saudi Arabia, and colleagues have synthesized seven air-stable gem-diaurated gold(III) complexes (general structure pictured) from 1,3-diketones and tetrachloroauric acid (HAuCl4). The team used 2,6-di-tert-butylpyridine (DtBP) as a base and dichloromethane (DCM) and methanol as solvents.
The desired complexes were obtained in yields of 26–69 %. All complexes are air-stable solids and were characterized using single-crystal X-ray diffraction. The team found long Au–Au distances and no evidence for stabilization by aurophilic interactions. The catalytic properties of the complexes were tested, and the team found that the soluble derivatives are active, e.g., in the cycloisomerization of an N-propargylcarboxamide.
- Gem-Diaurated Gold(III) Complexes: Synthesis, Structure, Aurophilic Interaction, and Catalytic Activity,
Jonas F. Wunsch, Lukas Eberle, Joseph P. Mullen, Frank Rominger, Matthias Rudolph, A. Stephen K. Hashmi,
Inorg. Chem. 2022.
https://doi.org/10.1021/acs.inorgchem.1c03479