Tetrapyrrole macrocycles and their transition-metal complexes are important for various biological systems. Their unique structures and reactivities make tetrapyrrole complexes an interesting target in chemical synthesis.
Due to the rigid orientation of the ligands, tetrapyrrole complexes containing hydrocarbon ligands can favor radical reactions over β-H-elimination, enabling otherwise rare reaction pathways. A biological example for such a complex is vitamin B12, one of the few biologically essential molecules containing a direct metal–carbon bond. In organoiron chemistry, corrole ligands with various substitution patterns are frequently used, and the alkylation of such complexes containing an iron(III) center has been described previously. Iron(IV) analogues, however, are scarce.
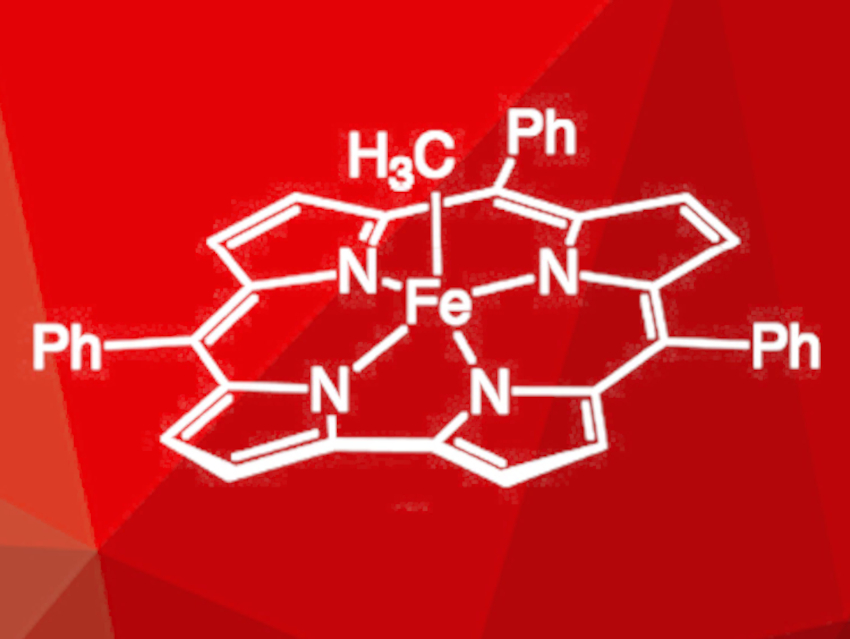
Zachary J. Tonzetich and Kenneth P. Caulfield, University of Texas at San Antonio, USA, have synthesized a series of alkylated iron(IV) triphenylcorrole (TPC) complexes of the type [Fe(R)(TPC)] (R = Me, Et, i-Pr, Cy, t-Bu, 1-Ad; methyl derivative pictured). The precursor complex {K(thf)2}[Fe(TPC)] was reacted with a range of alkyl iodides with different steric bulk. This reaction with alkyl iodides in diethyl ether gave the alkylated complexes swiftly. Alkylation using alkyl bromides was also possible, but proceeded more slowly, while the use of alkyl chlorides did not lead to the desired products.
The complexes with smaller alkyl groups were stable enough for isolation, while the bulkier complexes gradually decomposed within hours. Decomposition occurred via homolytic breaking of the Fe–C bond and hydrogen loss, yielding alkenes. When the ethyl-substituted complex decomposed in the presence of air, acetaldehyde was formed, while the propyl derivative gave acetone. When the complexes were treated with CO, no CO insertion was observed, while nitric oxide (NO) replaced the alkyl ligand. This indicates a preference for radical reactions due to homolysis of the Fe–C bond.
- Alkyl Complexes of Iron(IV) Triphenylcorrole,
Kenneth P. Caulfield, Zachary J. Tonzetich,
Organometallics 2022.
https://doi.org/10.1021/acs.organomet.1c00635