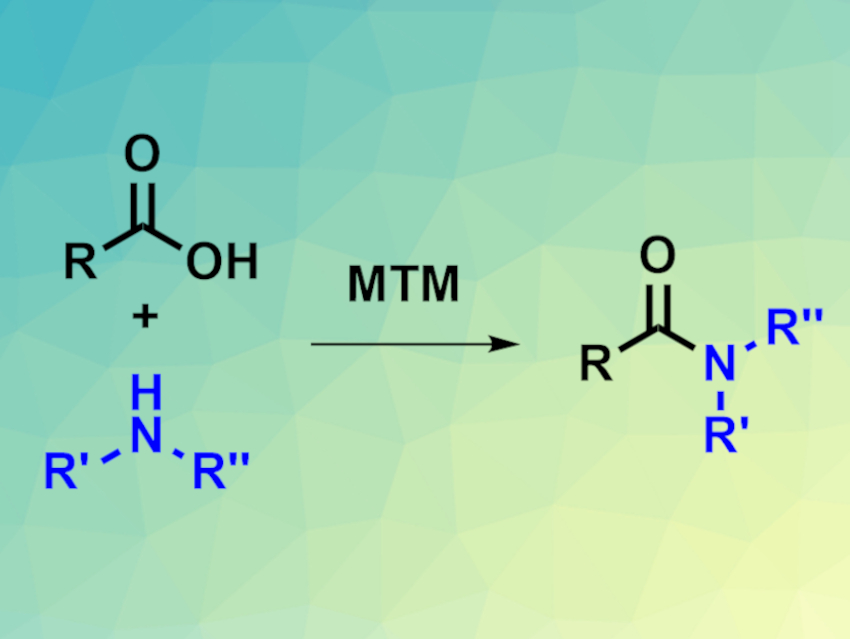
Amides are useful compounds, e.g., in the pharmaceutical industry. The direct amidation of carboxylic acids with amines is one approach for the synthesis of amides. A direct amidation reagent that is sustainable, non-toxic, as well as inexpensive, and provides high yields for a large substrate scope would be useful. Tetramethylorthosilicate (TMOS, Si(OMe)4), for example, can be used for such reactions. It is inexpensive and can be easily removed in a simple aqueous workup procedure, in which it is converted to silica. However, TMOS is harmful to health if it hydrolyzes to silica in the lungs.
D. Christopher Braddock, Joshua J. Davies, and Paul D. Lickiss, Imperial College London, UK, have found an alternative silicon-based reagent for direct amidations (pictured) that cannot undergo hydrolysis to silica but is still easy to remove after the reaction, namely, methyltrimethoxysilane (MTM, MeSi(OMe)3). MTM proved just as effective as TMOS for mediating many amidation reactions with primary amines, acyclic or cyclic secondary amines, and anilines in toluene as a solvent. The desired amides were obtained in moderate to excellent yields.
The team developed a workup procedure that provides the desired amides without a need for further purification. First, evaporation was used to remove the solvent as well as the siloxane and methanol byproducts formed from MTM. Nonvolatile polysiloxanes, methyl ester side products, and unreacted carboxylic acids were then removed by stirring the residue in a tetrahydrofuran (THF)/aqueous NaOH solution, giving the amide products in pure form. Alternatively, some secondary amide products crystallized from the reaction mixtures upon cooling, which allowed their isolation directly by filtration.
The team proposes a reaction mechanism in which the carboxylic acid reacts with MTM to produce a silyl ester and methanol, followed by an attack by the amine to form the amide product.
- Methyltrimethoxysilane (MTM) as a Reagent for Direct Amidation of Carboxylic Acids,
D. Christopher Braddock, Joshua J. Davies, Paul D. Lickiss,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.1c04265