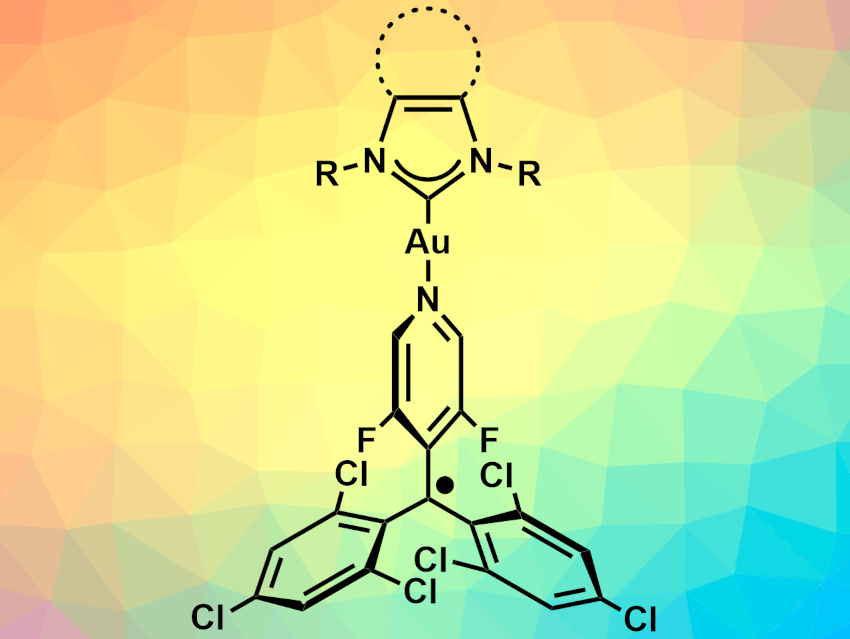
Radical fluorescence can be useful in the development of luminescent materials. Metal complexation can enhance the photoproperties of organic radicals. For example, the fluorescence quantum yield of the (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical (PyBTM) can be enhanced from 2 % to 8 % by coordination to a gold(I) phosphine complex [1].
Yohei Hattori, Ryukoku University, Otsu, Shiga, Japan, and colleagues have used a related, fluorine-functionalized radical ligand, the (3,5-difluoro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical (F2PyBTM), and enhanced its fluorescence quantum yield up to 36 % by coordination to Au(I) complexes with different N-heterocyclic carbene (NHC) ligands (general structure pictured). This is a new record fluorescence quantum yield among luminescent radical–metal complexes.
The team synthesized hexafluoroantimonate salts of the desired gold(I) complexes by reacting [Au(NHC)Cl]-type precursors with silver hexafluoroantimonate (AgSbF6) and F2PyBTM, giving the desired products and AgCl. The resulting radical complexes are stable under ambient conditions and have fluorescence quantum yields of 13–36 %. The highest fluorescence quantum yield was achieved using an 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (IPr) NHC ligand, which provides a nine-fold enhancement over the F2PyBTM radical alone.
- Amplification of luminescence of stable radicals by coordination to NHC–gold(I) complex,
Yohei Hattori, Ryota Kitajima, Ryota Matsuoka, Tetsuro Kusamoto, Hiroshi Nishihara, Kingo Uchida,
Chem. Commun. 2022.
https://doi.org/10.1039/d1cc06555f
Reference
- [1] Enhanced Luminescent Properties of an Open-Shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl Radical by Coordination to Gold,
Yohei Hattori, Tetsuro Kusamoto, Hiroshi Nishihara,
Angew. Chem. Int. Ed. 2015, 54, 3731–3734.
https://doi.org/10.1002/anie.201411572