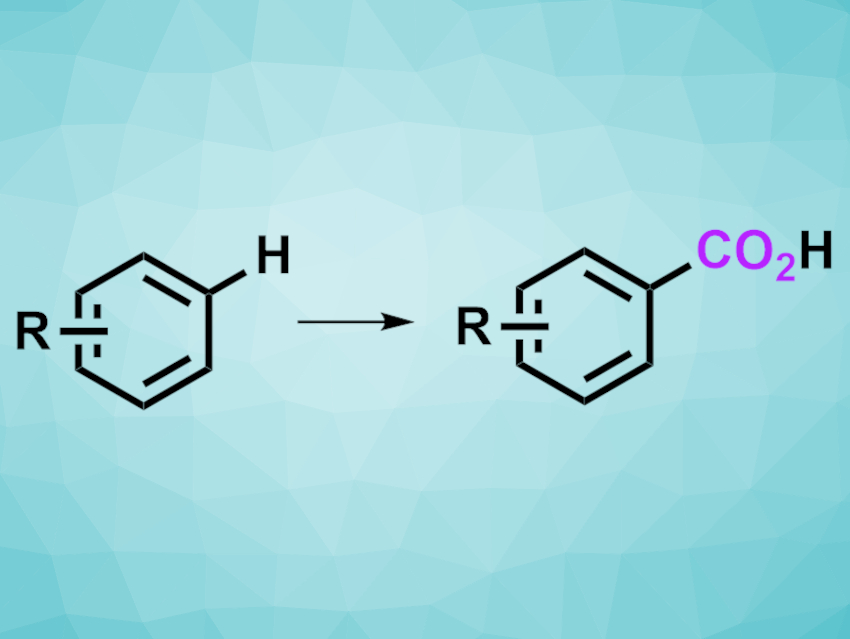
Aromatic carboxylic acids are useful building blocks in organic synthesis. Direct C–H bond carboxylations of aromatic compounds using abundant and nontoxic CO2 can be particularly useful paths to such carboxylic acids. However, existing approaches for this type of reaction have limited substrate scopes and functional group tolerances. Thus, developing new protocols with an improved range of possible substrates would be useful.
Masanori Shigeno, Yoshinori Kondo, Tohoku University, Sendai, Japan, and colleagues have used combined Brønsted base systems for aromatic C–H carboxylations with CO2. The team used LiOtBu or LiOCEtMe2 together with CsF and 18-crown-6 as a base/additive system and 1,3-dimethyl-2-imidazolidinone (DMI) as the solvent under a CO2 atmosphere. With this approach, they converted a variety of functionalized arenes to the corresponding carboxylic acids, which were methylated using MeI and obtained in the form of methyl esters.
The desired products were obtained in moderate to good yields. A wide range of functional groups was tolerated, including halogens, amides, nitriles, nitro groups, sulfones, sulfoxides, sulfonamides, ketones, and esters. The resulting polyfunctionalized aromatic carboxylic acid derivatives can be useful products or intermediates for further transformations.
- Direct C–H Carboxylation Forming Polyfunctionalized Aromatic Carboxylic Acids by Combined Brønsted Bases,
Masanori Shigeno, Kazuya Hanasaka, Itsuki Tohara, Koki Izumi, Hiroyuki Yamakoshi, Eunsang Kwon, Kanako Nozawa-Kumada, Yoshinori Kondo,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.1c03866