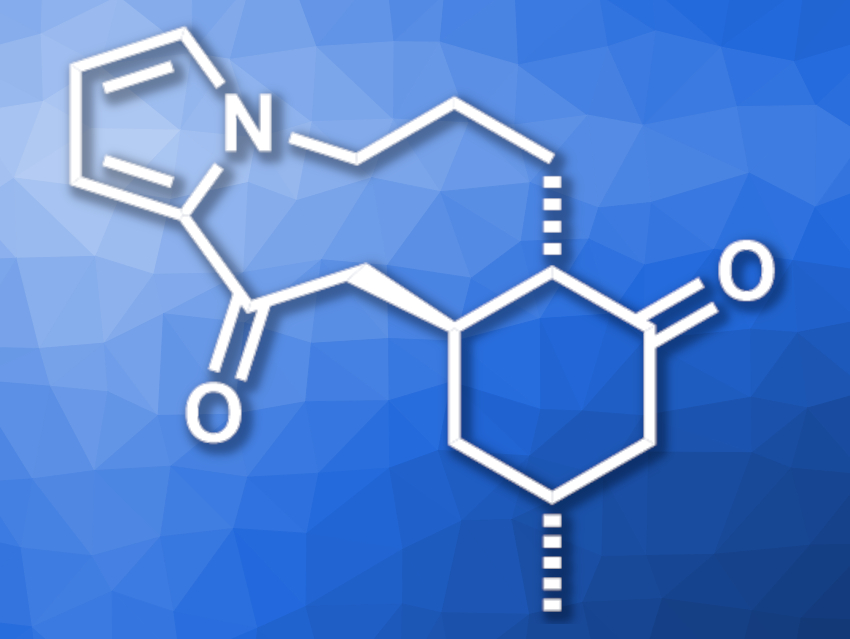
Huperzine H (pictured) is an alkaloid that was first isolated from Huperzia serrata, a type of clubmoss. It has a tricyclic skeleton with a six-membered ring, a nine-membered ring, and a pyrrole unit. The compound has three stereogenic centers, whose configurations had not been established so far.
Hayato Ishikawa, Chiba University, Japan, and colleagues have performed the first asymmetric total synthesis of huperzine H. The team started from commercially available (+)-pulegone, which was converted to an enone in four steps. The enone intermediate was then coupled with a silyl enol ether derivative of pyrrole in a Mukaiyama–Michael reaction. The resulting adduct was further functionalized to obtain a cyclization precursor, from which the nine-membered ring was formed. Finally, dehalogenation and desilylation reactions gave huperzine H.
The desired product was obtained in ten steps and with an overall yield of 12.1 %. Based on the asymmetric synthesis and X-ray diffraction analysis, the team was able to assign the relative and absolute stereochemistry of huperzine H.
- Asymmetric Total Synthesis and Structure Elucidation of Huperzine H,
Shinya Shiomi, Kaewsri Wilailak, Wataru Soutome, Hiromitsu Takayama, Mariko Kitajima, Hayato Ishikawa,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.1c02672


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
