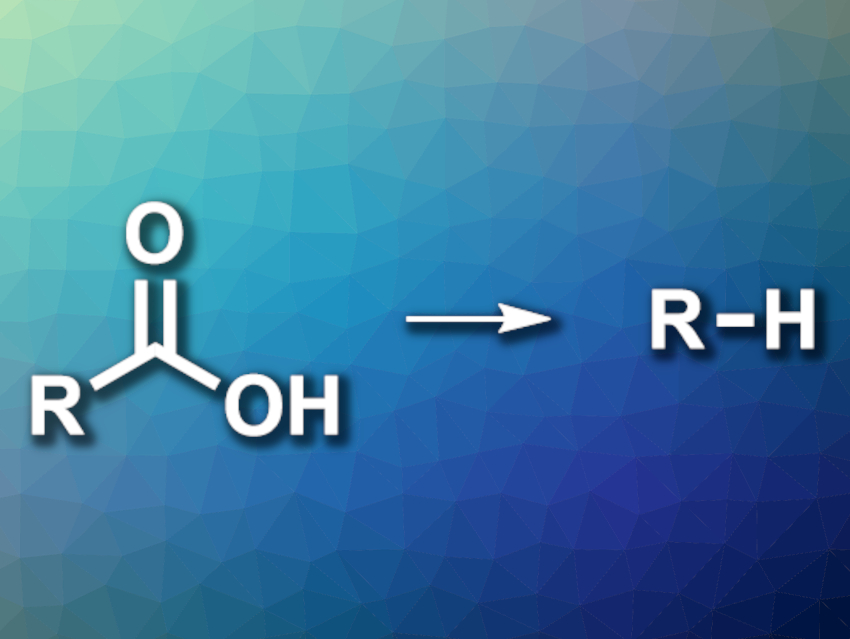
Carboxylic acids can be useful intermediates in organic synthesis. The removal of carboxylic acid groups after their use in a synthesis can be achieved by hydrodecarboxylation reactions (pictured). Existing methods for this type of reaction can have drawbacks such as a need for multiple steps, harsh reaction conditions, or harmful reagents, as well as issues with scalability.
Ai-Lan Lee, Heriot-Watt University, Edinburgh, UK, and colleagues have developed a simple, metal- and light-free, easy-to-scale method for the hydrodecarboxylation of aliphatic carboxylic acids. The team used primary, secondary, and tertiary carboxylic acids as substrates and reacted them with (NH4)2S2O8 using 2,4,6-collidine as a base and dimethyl sulfoxide (DMSO) as the solvent. The reactions were performed at 60 °C.
The desired products were obtained in moderate to good yields. Tertiary carboxylic acids are particularly well-suited substrates for the reaction, with yields up to 93 %. The team was able to perform the reaction on a gram scale, and the method showed a wide substrate scope and good functional group tolerance.
- Direct Hydrodecarboxylation of Aliphatic Carboxylic Acids: Metal- and Light-Free,
Euan B. McLean, David T. Mooney, David J. Burns, Ai-Lan Lee,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.1c04079