Chiral organophosphorus compounds, e.g., phosphines or phosphonates, are useful as ligands in asymmetric organic synthesis and in pharmaceutical chemistry, for example. However, the enantioselective synthesis of these compounds remains challenging and often requires stoichiometric amounts of chiral auxiliaries or gives racemates that require optical resolution.
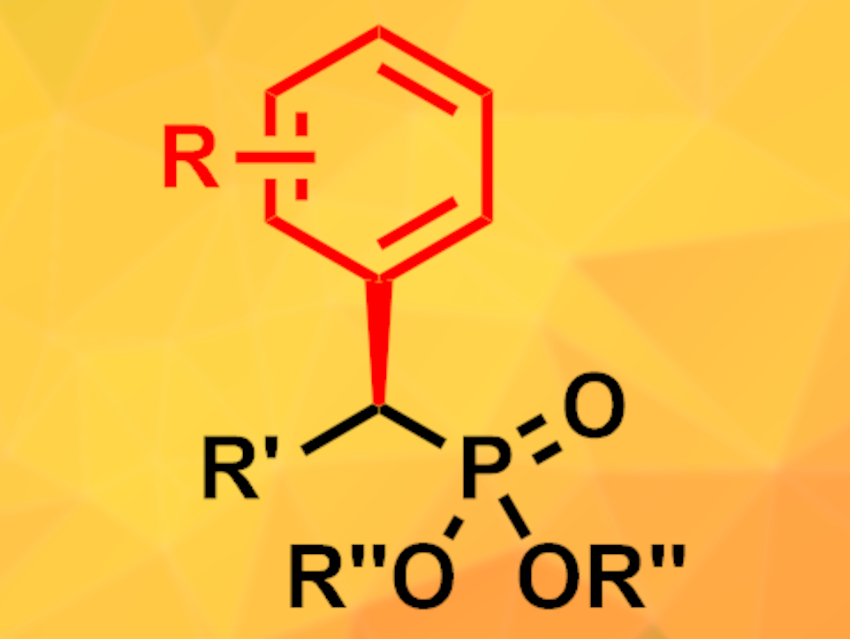
Yuqiang Li, Central South University, Changsha, China, Tao Xu, Tongji University, Shanghai, China, and colleagues have developed a cross-coupling reaction for the synthesis of chiral organophosphorus compounds (example pictured) using a combination of nickel- and photoredox catalysis. The phosphorus component, an α-brominated phosphonate or phosphine oxide, was coupled with an aryl iodide in a mixture of dioxane and dimethylacetamide under blue LED light. The reaction was catalyzed by a nickel complex made from NiBr2(DME) (DME = dimethoxyethane) and a chiral imidazoline-based ligand, as well as the commercially available photocatalyst 4CzIPN (2,4,5,6-tetrakis(9H-carbazol-9-yl) isophthalonitrile). Cesium carbonate was used as a base and Hantzsch ester as an additive.
The reaction gives the desired products in moderate to excellent yields and with high enantioselectivities. The highest yields were achieved with aryl iodides carrying electron-withdrawing groups. Different alkoxy groups at the phosphonate were tolerated. The reaction is compatible with reactive functional groups, such as aldehydes, as well as heterocyclic aryl iodides, providing access to a wide range of chiral phosphonates. Phosphine oxides undergo the cross-coupling reaction with slightly lower yields and enantioselectivities. The reduction of the resulting products provides access to the corresponding phosphines, which can be useful ligands, in good yields.
The team found that the addition of the stable free radical TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxyl) inhibited the reaction, which indicates that the reaction follows a radical pathway. The researchers suggest a catalytic cycle starting with the oxidative addition of the aryl iodide to the Ni(0) catalyst, forming a Ni(II) species which is then oxidized to Ni(III) by an alkyl radical formed from the brominated starting material. The product is liberated by reductive elimination, resulting in a Ni(I) species, which is regenerated by photoredox catalysis.
- Modular and Facile Access to Chiral α-Aryl Phosphates via Dual Nickel- and Photoredox-Catalyzed Reductive Cross-Coupling,
Hepan Wang, Purui Zheng, Xiaoqiang Wu, Yuqiang Li, Tao XU,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c12424



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)