Multi-component reactions are one-pot reactions of more than two substrates, which lead to complex molecules without intermediate purification steps. This increases efficiency and enables greener processes because fewer solvents and other materials are needed.
Three-component reactions are relatively common, but increasing the number of components beyond three leads to challenges such as unwanted side reactions and large amounts of byproducts. A well-known, synthetically highly useful four-component reaction is the Ugi reaction between an aldehyde, an amine, a carboxylic acid, and an isocyanide to give α-acylaminoamides. Other four-component reactions usually include a cyclization step, yielding heterocyclic products. Procedures for the four-component synthesis of open-chain molecules are rare.
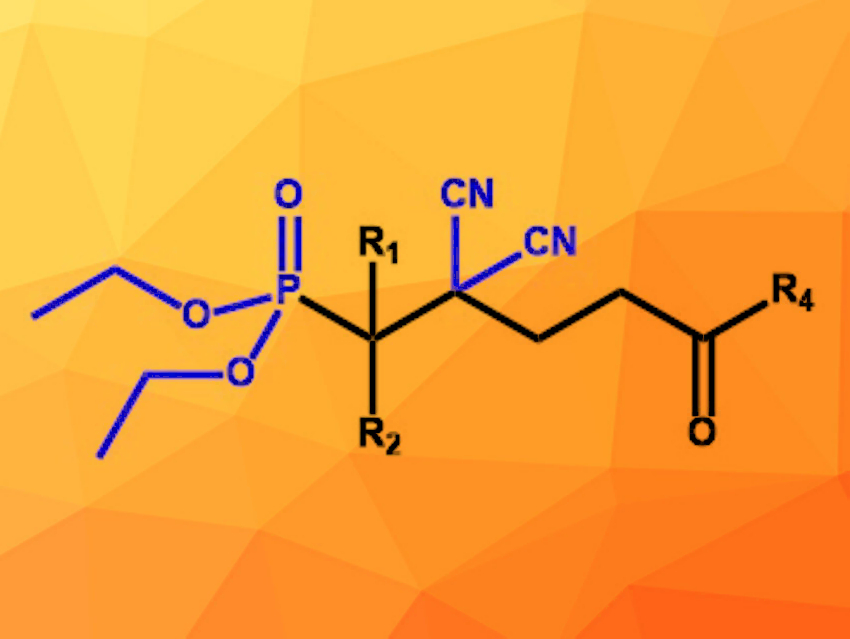
Ge Wang, Da-Zhen Xu, Nankai University, Tianjin, China, and colleagues have developed a four-component reaction for the synthesis of open-chain molecules with all-carbon, CN-substituted quaternary centers (example pictured). A carbonyl component, malononitrile (CH2(CN)2), an enone, and a nucleophile were reacted in THF at 40 °C. The ionic liquid [DABCO-H][AcO] (DABCO = 1,4-diazabicyclo[2.2.2]octane) was used as a catalyst. The desired products were formed in moderate to high yields up to 91 %.
The scope of carbonyl components includes various isatins and aromatic aldehydes with a wide variety of functional groups. The reaction is also compatible with different dialkyl phosphites as nucleophiles, but diethyl phosphite achieves the highest yields. Using 2-methylquinolines instead of the phosphites as nucleophiles also provided the desired products in good yields. The reaction offers access to a wide range of molecules containing different functional groups suitable for further conversions.
- Four-Component Reaction Access to Nitrile-Substituted All-Carbon Quaternary Centers,
Xin Hu, Qiang Bian, Zheng-Lin Wang, Lin-Jie Guo, Yi-Ze Xu, Ge Wang, Da-Zhen Xu,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c01863