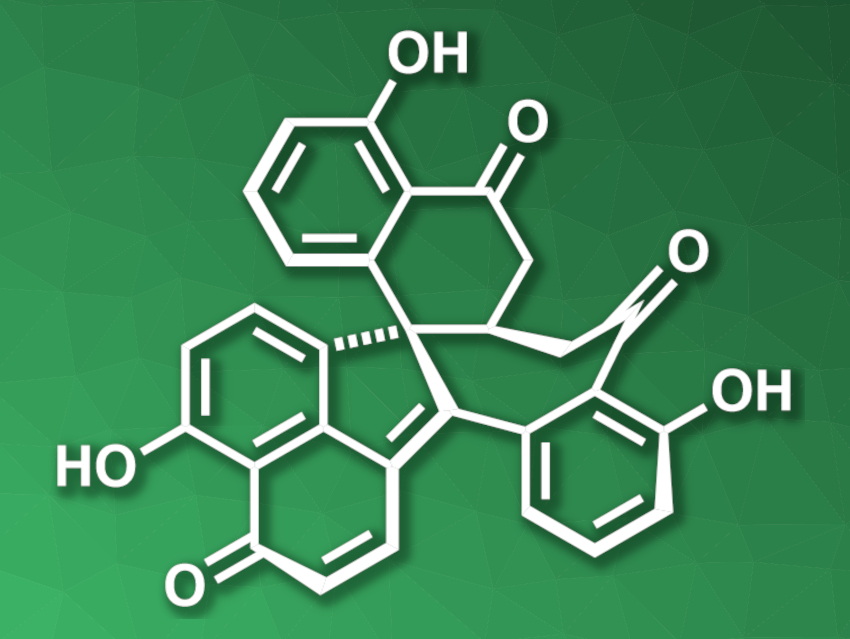
Dalesconol A (pictured) was initially isolated from fungi and shows biological activities, e.g., immunosuppressive activity and inhibition of acetylcholine esterase. The compound contains seven fused rings and is challenging to synthesize.
Xinjun Luan, Northwest University, Xi’an, and Nankai University, Tianjin, both China, and colleagues have developed a concise total synthesis of dalesconol A that uses a polycyclization/oxidation approach, i.e., the heptacyclic molecular skeleton is first assembled in one step and then the remaining oxygen functionalities are introduced. The team first prepared a naphthylamine-based fragment, which was reacted with a bromoalkyl alkyne with a protected ketone group via a palladium(0)/norbornene (NBE)-catalyzed three-fold domino reaction. This domino reaction was followed by a Michael addition to close the seventh ring and obtain the core of dalesconol A.
The resulting intermediate was oxidized in a stepwise manner, including a site-selective benzylic oxidation and the introduction of the three OH groups in one Pd(II)-catalyzed step. A final desaturation step then gave dalesconol A. The team also synthesized an amino analogue of dalesconol A from a heptacyclic intermediate using a transition-metal-catalyzed C–H amination reaction. Both dalesconol A and the amino analogue were synthesized in eight steps (longest linear sequence).
- Total Synthesis of Dalesconol A by Pd(0)/Norbornene-Catalyzed Three-Fold Domino Reaction and Pd(II)-Catalyzed Trihydroxylation,
Ping Zhao, Yun Guo, Xinjun Luan,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c12118