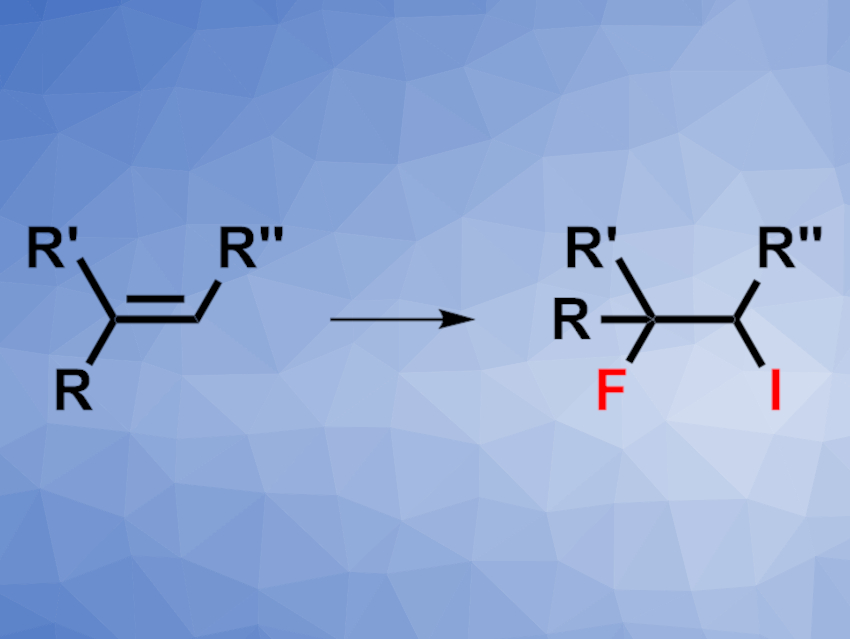
Fluorinated organic compounds are useful, e.g, in pharmaceutical or materials chemistry. Iodofluorinations of alkenes (pictured), for example, can be used to synthesize such compounds while introducing a synthetically useful iodine at the same time. Existing methods for the iodofluorination of alkenes to give 2-fluoroalkyl iodides often use, e.g., expensive iodonium cation sources or harmful F2 gas. A more convenient approach for this reaction would, thus, be useful.
Tsugio Kitamura, Saga University, Japan, and colleagues have developed a method for the iodofluorination of alkenes that uses elemental iodine and a HF·pyridine complex (pyr·9HF) to give 2-fluoroalkyl iodides under mild conditions. The team used K2S2O8 or Na2S2O8 as an oxidant and dichloromethane (DCM) as the solvent. The reactions were performed at room temperature.
The desired iodofluorinated products were obtained in moderate to high yields and with high regioselectivity. The team also successfully used the method on a gram scale. The products can be used as 2-fluoroalkyl building blocks for further reactions. The researchers propose a mechanism that involves the in-situ formation of iodine monofluoride (IF), which is then added to the alkene.
- Iodine-Mediated Fluorination of Alkenes with an HF Reagent: Regioselective Synthesis of 2-Fluoroalkyl Iodides,
Tsugio Kitamura, Ryuichi Komoto, Juzo Oyamada, Masahiro Higashi, Yosuke Kishikawa,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c02422