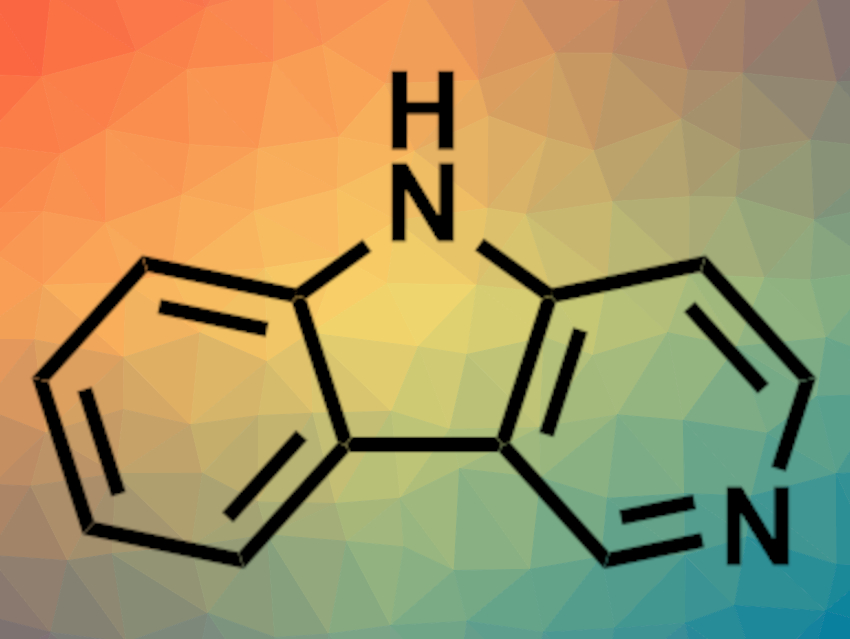
Carbolines are tricyclic compounds that consist of an indole unit and a pyridine ring. Such structures are found, e.g., in pharmaceutically active molecules. Methods for the synthesis of γ-carbolines (parent compound pictured) are limited, and the existing pathways generally require starting materials that have to be prepared in multiple steps.
Motohiro Sonoda, Shinji Tanimori, Osaka Prefecture University, Japan, and colleagues have developed a method for the synthesis of γ-carbolines using commercially available starting materials. The team started from 3,5-dibromo-4-pyridinamine, which was subjected to a monoarylation using a palladium-catalyzed Suzuki–Miyaura cross-coupling reaction. Then, a base-mediated ring closure with potassium tert-butoxide was used to form the central pyrrole ring.
The resulting γ-carbolines can be further functionalized, e.g., using another cross-coupling reaction. This can, for example, lead to compounds with fluorescent properties. The team also successfully used the approach for the synthesis of isocryptolepine, an alkaloid that shows interesting biological activities and contains a γ-carboline unit.
- Access to γ-Carbolines: Synthesis of Isocryptolepine,
Masahiro Akitake, Shizuki Noda, Kohei Miyoshi, Motohiro Sonoda, Shinji Tanimori,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c02026