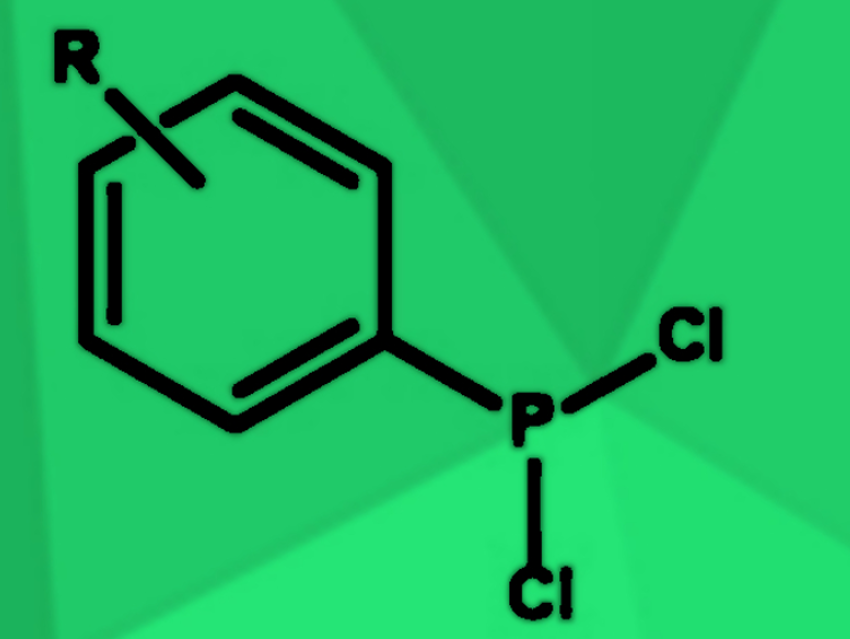
Dichlorophosphines (example pictured) are useful intermediates in the synthesis of many organophosphorus compounds. They are most commonly synthesized by reacting phosphorus trichloride (PCl3) with Grignard reagents or organolithium compounds. However, due to the high reactivity of these reagents, side reactions such as double or triple alkylations of the phosphorus center frequently occur.
This issue can be avoided if PCl(NEt2)2 is used as the phosphorus source instead of PCl3. The amino residues are substituted by hydrogen chloride after completion of the reaction, leading selectively to the dichlorophosphine. Drawbacks of this method are the use of hazardous HCl gas and the low atom economy due to the insertion and subsequent removal of the amino groups.
Konstantin Karaghiosoff, Ludwig-Maximilian University, Munich, Germany, and colleagues have developed an alternative route for the synthesis of aryl- and heteroaryl-dichlorophosphines using organozinc reagents. These reagents are less nucleophilic than Grignard or organolithium reagents and, therefore, less prone to side reactions. The starting aryl bromides were first lithiated using n-BuLi. After a transmetallation with ZnCl2, PCl3 was added. Volatile dichlorophosphines were isolated by distillation, non-volatile compounds by recrystallization. The desired products were obtained in moderate to high yields of 39–87 %.
The reaction procedure is compatible with a wide range of functional groups at the aryl bromide. Both electron-donating and electron-withdrawing para-substituents were tolerated, as well as bulky groups in ortho-position, polycyclic arenes, and heteroaromatics like thiophenes and furanes. The reaction can be carried out in a one-pot approach and uses readily available starting materials and reagents, while drawbacks of other methods like low selectivity or low atom economy are avoided.
- The Formation of P–C Bonds Utilizing Organozinc Reagents for the Synthesis of Aryl- and Heteroaryl-Dichlorophosphines,
Christin Kirst, Jonathan Tietze, Marian Ebeling, Lukas Horndasch, Konstantin Karaghiosoff,
J. Org. Chem. 2021.
https://doi.org/10.1021/acs.joc.1c01560