Stability data for pharmaceuticals is necessary for their approval. However, it can be challenging to predict the stability of pharmaceutical formulations, especially in the solid state. Prediction methods for the degradation of pharmaceutical ingredients in aqueous environments, e.g., using H2O2, may not give accurate results for this purpose and can require long reaction times.
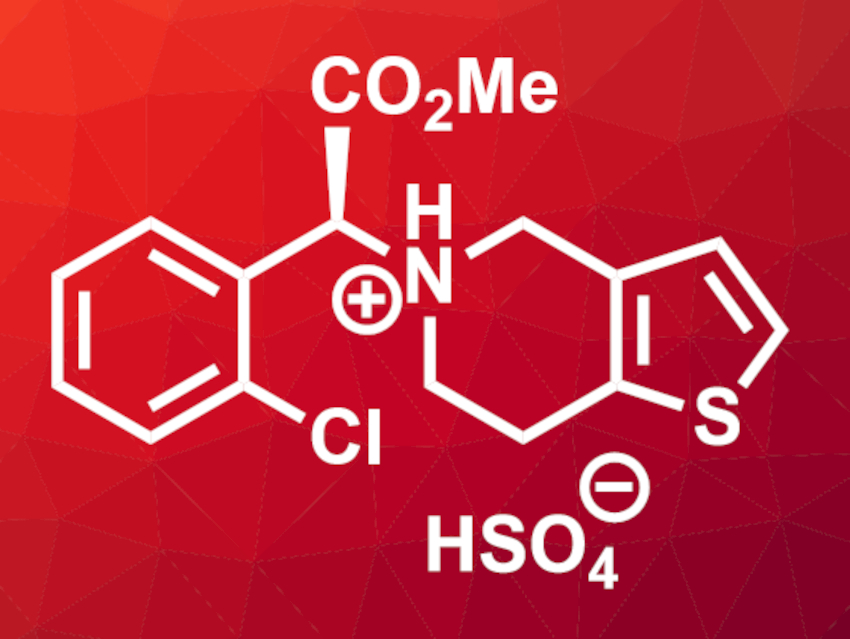
Torsten Beweries, Leibniz-Institut für Katalyse e.V., Rostock, Ulrike Holzgrabe, University of Würzburg, Carsten Bolm, RWTH Aachen University, all Germany, and colleagues have developed a method for the forced oxidative degradation of active pharmaceutical ingredients that uses ball milling. The team used clopidogrel hydrogensulfate as a model compound, an active ingredient that is used to prevent problems with blood clots, e.g., after heart attacks and strokes. They mixed the drug with one of three different oxidants (KMnO4, KNO3, or KHSO5·0.5 KHSO4·0.5 K2SO4) as well as inert SiO2 and ground the mixtures in a ball mill for 15 min.
Within this short reaction time, 30–40 % of the drug was degraded. The resulting residues were analyzed using high-performance liquid chromatography–mass spectrometry (HPLC–MS) as well as NMR and infrared (IR) spectroscopy. The team identified several degradation products, with the exact nature of the products depending on the used oxidant. The mechanochemical method is faster than existing approaches for predicting the degradation of active pharmaceutical ingredients and could provide more meaningful results for the solid state.
- Ball milling – a new concept for predicting degradation profiles in active pharmaceutical ingredients,
Reinhard P. Kaiser, Everaldo F. Krake, Laura Backer, Jonas Urlaub, Wolfgang Baumann, Norbert Handler, Helmut Buschmann, Torsten Beweries, Ulrike Holzgrabe, Carsten Bolm,
Chem. Commun. 2021.
https://doi.org/10.1039/d1cc04716g