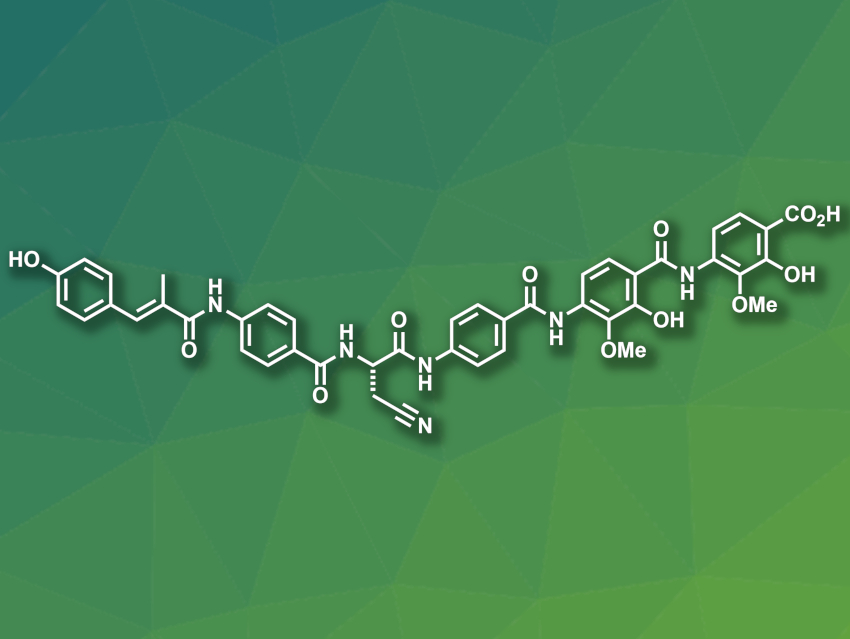
Multidrug-resistant bacteria increasingly pose problems in healthcare. New antibacterial agents that can target these microbes are, thus, urgently needed. Albicidin (pictured), for example, which was isolated from the bacterium Xanthomonas albilineans, is an antibacterial natural product. It is a hexapeptide containing several para-amino benzoic acid units. Optimizing the bioactivity of albicidin through changes in its structure could lead to a new antimicrobial drug.
Roderich D. Süssmuth, Technische Universität Berlin, Germany, and colleagues have synthesized a series of albicidin derivatives in which different phenyl groups are replaced with pyridine units. This exchange is commonly used in medicinal chemistry to improve the pharmacological properties of compounds. It can, e.g., improve solubility in water and allow for hydrogen bonding.

The team found a pyridine-containing derivative with promising antibacterial activity against different multidrug-resistant bacteria, e.g., Gram-negative E. coli and Gram-positive S. aureus. In mouse experiments, the compound showed a promising safety profile and good treatment efficiency in an E. coli infection model.
- Improvement of the antimicrobial potency, pharmacokinetic and pharmacodynamic properties of albicidin by incorporation of nitrogen atoms,
Lieby Zborovsky, Leonardo Kleebauer, Maria Seidel, Arseni Kostenko, Leonard von Eckardstein, Frank Otto Gombert, John Weston, Roderich D. Süssmuth,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc04019g