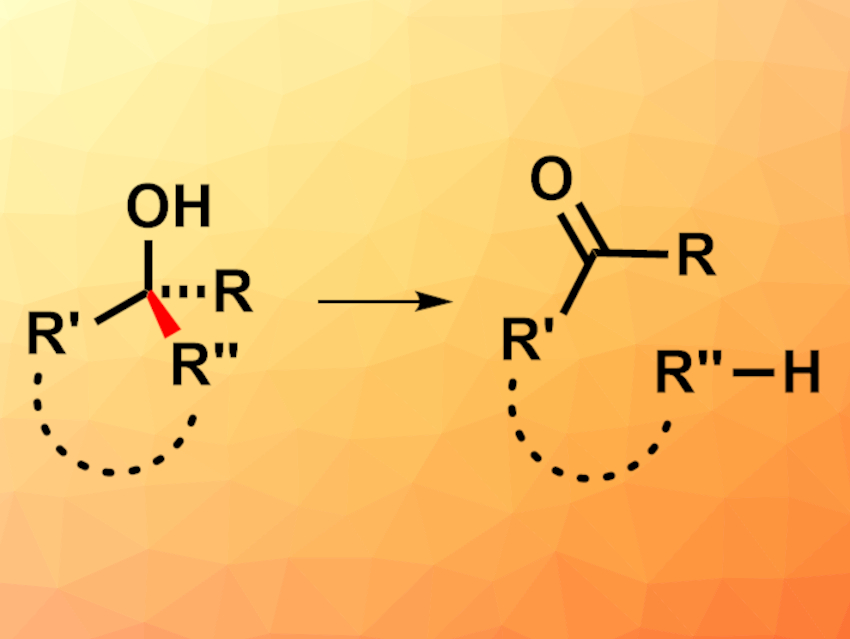
Organic syntheses often rely on C–C bond-forming reactions to create complex structures. However, strategically breaking a C–C bond can also be useful in synthesis. This can be challenging due to the low reactivity of C–C bonds.
Peng Hu, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed a visible-light-promoted, iron-catalyzed reaction of alcohols that gives ketones or aldehydes via a C–C bond cleavage (pictured). The team converted different cyclic or linear alcohols in the presence of FeCl3 as a catalyst, tetrabutylammonium chloride (TBACl) as a source of chloride ions, bis(2,4,6-triisopropylphenyl) disulfide (TRIP2S2) as a radical source, and 2,4,6-collidine to bind the formed HCl under a blue LED. They used tertiary, secondary, and primary alcohols with a wide range of substituents.
The desired products were obtained in moderate to excellent yields. The reaction has a broad substrate scope and good functional group tolerance. The researchers propose that the mechanism proceeds via a pathway promoted by a chlorine radical, which is generated from [FeCl4]− upon excitation by light. This chlorine radical reacts with the alcohol to generate an alkoxy radical, followed by the C–C bond breaking and a hydrogen-atom transfer to give the desired products.
- Iron-Catalyzed C–C Single-Bond Cleavage of Alcohols,
Wei Liu, Qiang Wu, Miao Wang, Yahao Huang, Peng Hu,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03137