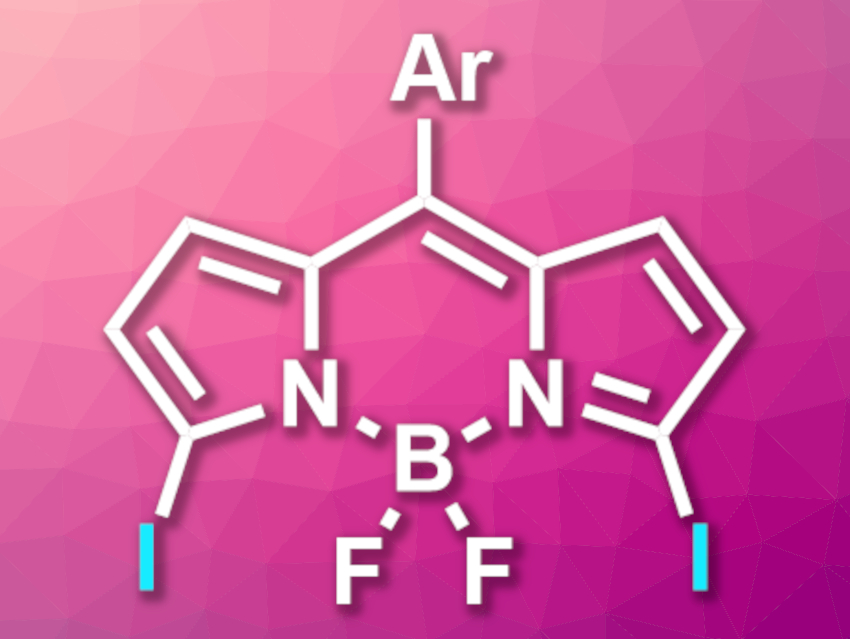
BODIPY, or 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, dyes are used as fluorophores with a wide range of applications. Methods for the synthesis of BODIPY derivatives are, thus, an interesting research target. Halogenated BODIPYs are useful intermediates in such syntheses. While 2,6-dichloro-, dibromo-, and diiodo-BODIPYs can be readily synthesized, 3,5-halogenated derivatives are more challenging to prepare.
Michael J. Hall, Julian G. Knight, Newcastle University, Newcastle Upon Tyne, UK, and colleagues have prepared 3,5-diiodo-8-aryl-BODIPYs (pictured) from the corresponding 3,5-dichloro- and 3,5-dibromo-derivatives. The team used a double aromatic Finkelstein reaction approach. They reacted the substrates with NaI, using propionitrile (EtCN) as a solvent.
The researchers obtained the desired diiodo-functionalized products in moderate to excellent yields. The 3,5-dibromo-functionalized substrates showed higher reaction rates than the dichloro-analogues. According to the team, this indicates that the reactions of 3,5-dibromo-BODIPYs proceed via a concerted SNAr mechanism. The diiodo-functionalized products can be further transformed in a variety of palladium-catalyzed cross-coupling reactions to create new BODIPY derivatives.
- Synthesis and Reactivity of 3,5-Diiodo-BODIPYs via a Concerted, Double Aromatic Finkelstein Reaction,
Felicity J. Frank, Paul G. Waddell, Michael J. Hall, Julian G. Knight,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03317