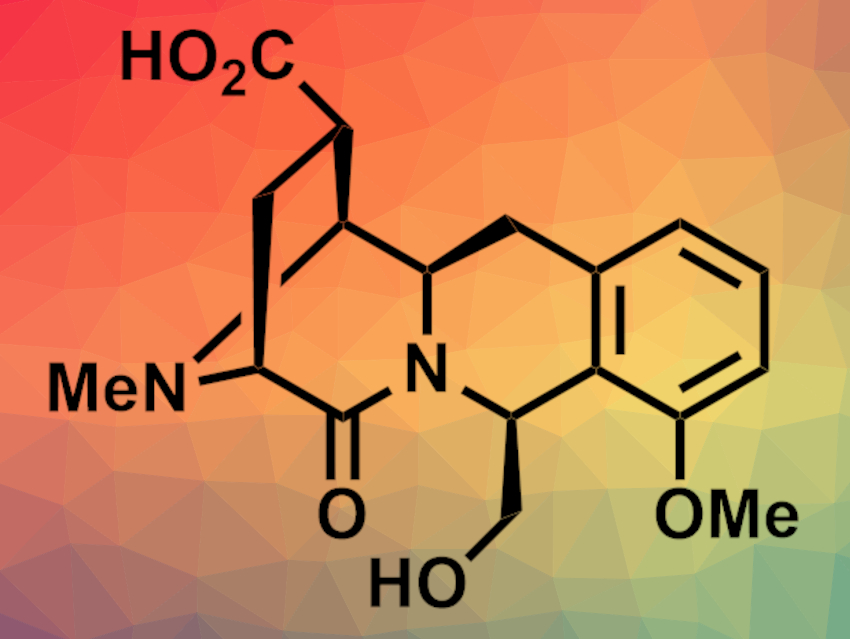
(−)-Quinocarcin is a pentacyclic alkaloid that has shown antitumor activities, which makes the compound and its derivatives interesting targets for total synthesis. Under anaerobic conditions, quinocarcin can disproportionate to give quinocarcinol and quinocarcinamide (pictured). No asymmetric total synthesis of the latter had been reported so far.
Kun Wei, Yu-Rong Yang, Kunming Institute of Botany, Chinese Academy of Sciences, and colleagues have performed the first asymmetric total synthesis of (+)-quinocarcinamide. The team started by preparing a racemic allylic alcohol from 2-bromo-6-methoxybenzaldehyde. An iridium-catalyzed asymmetric allylic amidation was used to convert this intermediate to a chiral allylic amide, which was subjected to ozonolysis to obtain a primary alcohol.
A Stille coupling and a cross-metathesis reaction were used to further build the carbon skeleton. The tetrahydroisoquinoline ring system was closed via a nucleophilic substitution and acylated to introduce the amide group. The diazabicyclo[3.2.1]octane ring was formed via a stereocontrolled 1,3-dipolar cycloaddition. According to the researchers, the strategy might also be useful for the synthesis of other tetrahydroisoquinoline alkaloids.
- Asymmetric Total Synthesis of (+)-Quinocarcinamide,
Lei Li, Li Shi, Kun Wei, Yu-Rong Yang,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02970