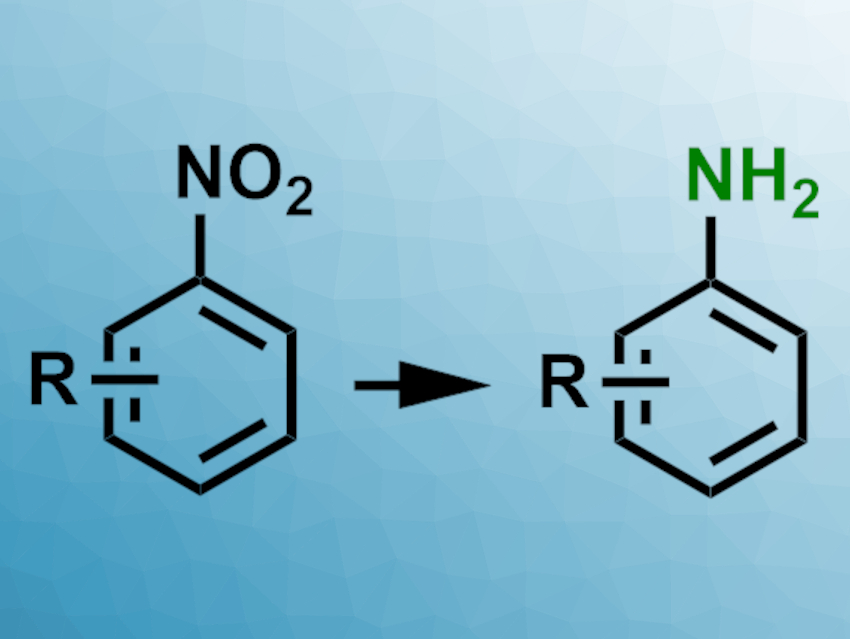
Many pharmaceutical intermediates and drug molecules contain amino-substituted aromatic groups. Since the nitration of aromatic molecules is generally easily achieved, aromatic amines are often synthesized via the reduction of nitro groups. However, this can require high catalyst loadings.
Balaram S. Takale, Bruce H. Lipshutz, University of California, Santa Barbara, USA, and colleagues have developed a procedure for the reduction of aromatic nitro groups to the corresponding amines that only requires 0.4 mol% of a Pd/C catalyst and is run in water under an atmospheric pressure of H2. The aqueous medium is mixed with 2 wt% of the “designer” surfactant TPGS-750-M. The surfactant forms nanomicelles in which the reaction of the usually not water-miscible substrates takes place. After the reaction, the product can be easily isolated by filtration or extraction, and the surfactant and the catalyst can be reused.
The reaction proved efficient for a wide range of functionalized aryl- and heteroaryl nitro compounds, including various important pharmaceutical intermediates, which were cleanly reduced to the corresponding amines in high yields. The reduction was highly selective, and reducible functional groups like nitriles or ketones, as well as common protecting groups, remained intact. The approach could also be extended to various one-pot synthesis routes for different pharmaceutical intermediates, e.g., with an SNAr reaction or acylation following the nitro reduction in the same reaction vessel.
- High Turnover Pd/C Catalyst for Nitro Group Reductions in Water. One-Pot Sequences and Syntheses of Pharmaceutical Intermediates,
Xiaohan Li, Ruchita R. Thakore, Balaram S. Takale, Fabrice Gallou, Bruce H. Lipshutz,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03258